Abstract
Background: Pandemics may promote hospital avoidance, and added precautions may exacerbate treatment delays for medical emergencies such as stroke. We sought to evaluate ischemic stroke presentations, management and outcomes during the first year of the COVID-19 pandemic.
Methods: We conducted a population-based study, using linked administrative and stroke registry data from Alberta to identify all patients presenting with stroke before the pandemic (Jan. 1, 2016 to Feb. 27, 2020) and in 5 periods over the first pandemic year (Feb. 28, 2020 to Mar. 31, 2021), reflecting changes in case numbers and restrictions. We evaluated changes in hospital admissions, emergency department presentations, thrombolysis, endovascular therapy, workflow times and outcomes.
Results: The study included 19 531 patients in the prepandemic period and 4900 patients across the 5 pandemic periods. Presentations for ischemic stroke dropped in the first pandemic wave (weekly adjusted incidence rate ratio [IRR] 0.54, 95% confidence interval [CI] 0.50 to 0.59). Population-level incidence of thrombolysis (adjusted IRR 0.50, 95% CI 0.41 to 0.62) and endovascular therapy (adjusted IRR 0.63, 95% CI 0.47 to 0.84) also decreased during the first wave, but proportions of patients presenting with stroke who received acute therapies did not decline. Rates of patients presenting with stroke did not return to prepandemic levels, even during a lull in COVID-19 cases between the first 2 waves of the pandemic, and fell further in subsequent waves. In-hospital delays in thrombolysis or endovascular therapy occurred in several pandemic periods. The likelihood of in-hospital death increased in Wave 2 (adjusted odds ratio [OR] 1.48, 95% CI 1.25 to 1.74) and Wave 3 (adjusted OR 1.46, 95% CI 1.07 to 2.00). Out-of-hospital deaths, as a proportion of stroke-related deaths, rose during 4 of 5 pandemic periods.
Interpretation: The first year of the COVID-19 pandemic saw persistently reduced rates of patients presenting with ischemic stroke, recurrent treatment delays and higher risk of in-hospital death in later waves. These findings support public health messaging that encourages care-seeking for medical emergencies during pandemic periods, and stroke systems should re-evaluate protocols to mitigate inefficiencies.
In response to the COVID-19 pandemic, affected countries implemented various public health measures to decrease viral transmission. An unintended consequence of these measures could be hospital avoidance by patients with medical emergencies, as observed during other outbreaks in the 2000s.1,2 Some public health messaging specifically warned groups at high cardiovascular risk, such as older people or those with heart disease, that they were at elevated risk of severe COVID-19.3 Physical distancing may also result in loss of services and support networks, impairing patients’ ability to seek medical assistance.4 Furthermore, pandemics generate new challenges of managing personal protective equipment and cleaning protocols,5 and additional information bottlenecks, which could result in workflow delays for emergencies like stroke.6
Previous studies have reported declines in patients presenting to hospital with stroke or acute coronary syndrome during the pandemic.7,8 A World Stroke Organization survey of members in several countries indicated a sharp reduction in stroke admissions by 50%–80% in the first weeks of the pandemic.9 A cross-sectional study reported a global decline in hospital admissions for stroke.10 Patients who present to hospital seem to be doing so later than usual, perhaps waiting until their condition becomes more severe.11–14 However, studies have not been at a population level, consequently suffering from selection bias, and have generally focused only on the first wave of the pandemic. As the associations between the pandemic and the incidence, treatment, workflow and outcomes of stroke are likely to be modified by several events — including changing COVID-19 case counts, public health restrictions and health system strains — it is important to explore population data from pandemic periods beyond the first wave to better understand these phenomena.
Verifying and quantifying the pandemic’s effect on stroke presentations and workflow can help tailor public health messaging to continue emphasizing the time-critical nature of emergencies like stroke. Such data may also help optimize pandemic stroke workflow protocols. We sought to explore patterns of hospital admissions, treatment rates, workflow delays and outcomes for ischemic stroke during the first year of the COVID-19 pandemic in Alberta, Canada.
Methods
Data sources
We used linked administrative and registry data capturing stroke-related information for the entire population of Alberta.15 We obtained data on all patients admitted to hospital with ischemic stroke during the prepandemic and pandemic periods of interest (defined below) from the Discharge Abstract Database, maintained by the provincial health authority (Alberta Health Services Analytics group via the Alberta Strategy for Patient-Oriented Research Support Unit,) which contains demographic and clinical information on hospital discharges, including comorbidities and need for continuing care (e.g., assisted living, nursing home care).16 In addition, we obtained data on all emergency department visits for minor stroke (i.e., patients presenting with ischemic stroke who were discharged home directly from the emergency department) in Alberta from the National Ambulatory Care Reporting System. These data are reliable in identifying ischemic stroke and vascular risk factors;17 codes for stroke from the International Classification of Diseases, 10th Revision (Appendix 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.211003/tab-related-content) are 92%–97% accurate.18
We extracted data on use of thrombolysis and endovascular therapy through the Quality Improvement and Clinical Research Program, which includes 2 comprehensive stroke centres (sole providers of endovascular therapy) and the 15 urban and remote hospitals designated as Alberta’s primary stroke centres, which can provide thrombolysis. No other centres provide hyperacute stroke care. This registry, released in 2016, is managed by stroke coordinators at each of the 17 stroke centres. Quality control measures are taken quarterly; the data are comprehensively validated against electronic medical records. We abstracted patient demographics, stroke severity (National Institutes of Health Stroke Scale score),19 prehospital workflow (time of stroke onset and of hospital arrival, “onset-to-door”) and in-hospital workflow (computed tomography [CT], thrombolysis, endovascular therapy, groin puncture and reperfusion times). These data were linked using provincial health care numbers.
Independent of our main study population, we used vital statistics data from Alberta Health Services on all deaths attributed to stroke in the province during the study period. We used these data to determine the proportion of out-of-hospital deaths among all stroke deaths, capturing patients who did not receive stroke care in the pandemic periods compared with the prepandemic period.
Study period
Alberta’s first COVID-19 case was identified on Feb. 28, 2020, and public health restrictions were implemented shortly afterwards; 20 therefore, we defined the prepandemic period as Jan. 1, 2016 to Feb. 27, 2020. We divided the pandemic period into 5 parts (Wave 1, lull period, Wave 2.1, Wave 2.2 and Wave 3) based on key changes in reported COVID-19 case counts, timing of public health restrictions and impact on delivery of health services. The key events that distinguished these time periods are detailed in Appendix 1. Wave 1 (Feb. 28, 2020 to May 12, 2020) was characterized by a relatively small number of cases, with a peak in new daily cases on Apr. 29, 2021 (315 new cases and a 7-day rolling average of 252 new cases), but with public health restrictions in full force. A relative lull period (May 13, 2020 to July 20, 2020) began with a gradual relaunch strategy and was characterized by a relative flattening of the curve. Wave 2.1 (July 21, 2020 to Oct. 11, 2020) was the first part of Alberta’s long second wave, which initially peaked at 277 new daily cases and a rolling average of 234 cases. Wave 2.2 (Oct. 12, 2020 to Feb. 15, 2021), the second part of Wave 2, was characterized by a major inflection after Thanksgiving gatherings, with new daily cases peaking at 1887 on Dec. 14, 2020. During this period, the contact tracing system became overwhelmed and the province returned to lockdown-style restrictions. Wave 3 (Feb. 16, 2021 to Mar. 31, 2021) began as new SARS-CoV-2 variants of concern arrived, coinciding with partial reopening of the province. Case counts rose from a trough of 251 new cases per day. Data are complete until Mar. 31, 2021, during which Wave 3 was ongoing.
Statistical analysis
For the primary analyses, we used Poisson regression (adjusted for age, sex, continuing care needs and comorbidities) to compare the rate of patients presenting to emergency departments with stroke, and the rate of thrombolysis and endovascular therapy for stroke. For the incidence rate ratio calculations in the Poisson regressions, we used weekly rates (e.g., hospital admissions or presentations for stroke per week for each of the 5 pandemic periods, compared with the prepandemic period), and then verified our findings using monthly and quarterly rates. We also plotted unadjusted data for weekly presentations for ischemic stroke and monthly utilization rates of thrombolysis and endovascular therapy, with trendlines generated from interrupted time-series analyses, specifying knots (i.e., potential slope changes) at the start of each time period of interest.
For secondary analyses of prehospital factors, we compared the proportion of all patients presenting with stroke who received thrombolysis and endovascular therapy in each of the 5 pandemic periods with the prepandemic period using logistic regression, adjusted for age, sex, preadmission continuing care needs and comorbidities. We used similar logistic regressions to compare proportions of in-hospital deaths, and of all deaths attributed to stroke that occurred out of hospital. We compared preand in-hospital workflow times and hospital length of stay using quantile regressions (chosen instead of linear models given nonnormality of residuals), adjusted for age, sex, continuing care needs and comorbidities. We compared scores on the National Institutes of Health Stroke Scale at admission in each pandemic period with the prepandemic period using quantile regressions, adjusted for age, sex, continuing care needs and comorbidities.
We stratified analyses of incidence and workflow times by age (≥ 65 v. < 65 yr), comorbidities (any v. none) and preadmission continuing care needs (any v. none) after testing for heterogeneity of effects, as we were particularly interested if there were differences among these groups. All analyses were performed with STATA/MP 16.1. We defined statistical significance as p less than 0.05.
Ethics approval
The study was approved by the University of Calgary Conjoint Health Research Ethics Board (REB20–0769). No informed consent was required.
Results
Among the 24 431 patients with ischemic stroke that we included, 19 531 presented before the pandemic and 4900 presented during the pandemic (Table 1). Patients discharged without admission after a minor stroke constituted a smaller proportion of all ischemic strokes during the pandemic, particularly in Wave 1 (age- and sex-adjusted odds ratio [OR] 0.76, 95% confidence interval [CI] 0.63 to 0.91) and Wave 2.2 (adjusted OR 0.86, 95% CI 0.76 to 0.98). Patients who presented during the pandemic were less likely to have atrial fibrillation in any pandemic period, to have hypertension in Wave 1 and the lull period, and to have 2 or more comorbidities in Wave 1, compared with the prepandemic period. However, patients were more likely to have coronary artery disease in Waves 2.2 and 3, and more likely to have 1 or more comorbidities in Wave 2.2 than in the prepandemic period (adjusted OR 1.25, 95% CI 1.12 to 1.39).
Baseline characteristics of patients presenting with ischemic stroke before (Jan. 1, 2016 to Feb. 27, 2020) and during (Feb. 28, 2020 to Mar. 31, 2021) the first year of the COVID-19 pandemic in Alberta, Canada
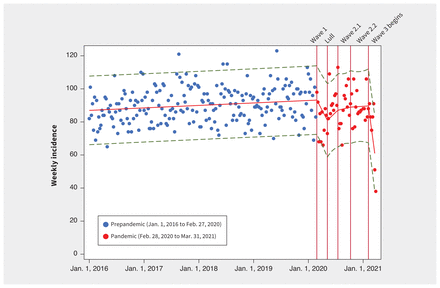
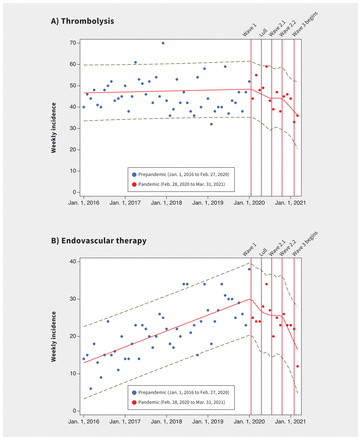
Hospital admissions and presentations for ischemic stroke decreased in Wave 1 compared with the prepandemic period, whether we used weekly, monthly or quarterly epochs (Table 2). Although the incidence increased in the lull period, it did not return to prepandemic levels, and the incidence declined further by Wave 3 (Figure 1). We found no interaction by age, comorbidities, previous care needs, or minor or major stroke diagnoses. We observed similar declines in the incidence of thrombolysis (Figure 2A) and endovascular therapy (Figure 2B, Table 2), compared to the prepandemic period, during which the overall incidence of thrombolysis was relatively stable and the use of endovascular therapy was increasing. However, the proportion of patients presenting with ischemic stroke who were treated with thrombolysis or endovascular therapy did not change significantly (Table 3). We found the odds of endovascular therapy were higher during Wave 2.2 (when case numbers peaked) than in the prepandemic period (adjusted OR 1.33, 95% CI 1.08 to 1.64).
Change in the incidence of hospital admissions and presentations, and of acute therapies for ischemic stroke during the first year of the COVID-19 pandemic (Feb. 28, 2020 to Mar. 31, 2021), compared with before the pandemic (Jan. 1, 2016 to Feb. 27, 2020) in Alberta, Canada
Weekly incidence of hospital admissions and presentations for ischemic stroke in Alberta, Canada before and during the first year of the COVID-19 pandemic. We defined the prepandemic period as Jan. 1, 2016 to Feb. 27, 2020, and the pandemic period as Feb. 28, 2020 to Mar. 31, 2021 (divided into 5 periods: Wave 1, lull period, Wave 2.1, Wave 2.2 and Wave 3). The x-axis is marked off in 1-year increments. Trendlines are from an interrupted time-series analysis, with knots specified at transitional time points from 1 study period to the next. The broken lines represent 95% confidence intervals.
Monthly incidence of (A) thrombolysis and (B) endovascular therapy for ischemic stroke in Alberta, Canada before and during the first year of the COVID-19 pandemic. We defined the prepandemic period as Jan. 1, 2016 to Feb. 27, 2020, and the pandemic period as Feb. 28, 2020 to Mar. 31, 2021 (divided into 5 periods: Wave 1, lull period, Wave 2.1, Wave 2.2 and Wave 3). The x-axis is marked off in 1-year increments Trendlines are from an interrupted time-series analysis, with knots specified at transitional time points from 1 study period to the next. The broken lines represent 95% confidence intervals.
Acute treatments and outcomes among patients presenting or admitted to hospital with ischemic stroke, and out-of-hospital stroke deaths before (Jan. 1, 2016 to Feb. 27, 2020) and during (Feb. 28, 2020 to Mar. 31, 2021) the first year of the COVID-19 pandemic in Alberta, Canada
Onset-to-door times among treated patients were notably prolonged during the lull period (adjusted difference 24.3 min, 95% CI 9.1 to 39.4 min, Table 4). This effect was not modified by age or continuing care needs. Compared with the prepandemic period, recipients of endovascular therapy had delayed times from CT to groin puncture and from door to groin puncture in Waves 1 and 2.2; prolonged times from groin puncture to reperfusion during Wave 1, the lull period and Wave 2.2; and delayed door-to-reperfusion times during Wave 1 (adjusted difference 29.1 min, 95% CI 3.5–54.7 min) and the lull period (adjusted difference 29.2 min, 95% CI 4.6 to 53.7 min). However, door-to-CT times were shorter during Wave 2.2 (adjusted difference −1.8 min, 95% CI −3.5 to −0.05 min).
Prehospital and in-hospital workflow and stroke severity of patients with ischemic stroke who received acute stroke therapies before (Jan. 1, 2016 to Feb. 27, 2020) and during (Feb. 28, 2020 to Mar. 31, 2021) the first year of the COVID-19 pandemic in Alberta, Canada
Among patients treated with thrombolysis or endovascular therapy, there were no overall differences in stroke severity at baseline, except in Wave 1, when recipients of endovascular therapy had significantly lower scores on the National Institutes of Health Stroke Scale (Table 4).
The proportion of all stroke deaths in Alberta that occurred out of hospital increased significantly during all pandemic periods except Wave 2.1 (Table 3). The incidence of in-hospital death was markedly higher in Wave 2.2 and Wave 3, even after adjusting for age, sex, continuing care needs before stroke and comorbidities (Table 3). The relation of the study period to in-hospital death was significantly modified by receipt of acute stroke therapies in Wave 2.1, during which in-hospital deaths were lower among treated patients (pinteraction = 0.01; Appendix 1, Supplementary Table 1). Length of hospital stay did not generally change in the pandemic periods except in Wave 3, when they were shorter (adjusted difference −2.0 d, 95% CI −3.1 to −0.9 d).
Interpretation
In the population of a Canadian province, we found that the first wave of the COVID-19 pandemic was associated with a marked decline in rates of patients presenting to hospital with ischemic stroke and in the absolute use of acute stroke therapies, which did not return to prepandemic levels in subsequent waves during the first year of the pandemic. We also observed delays in presentation to hospital in the early pandemic period, recurrent delays in in-hospital workflow and higher odds of in-hospital death attributed to stroke in the second and third waves.
Our findings validate concerns raised from survey data about the pandemic’s effects on medical emergencies during the first wave.21,22 Declines of 40% or greater in admissions for myocardial infarction and stroke were reported from surveys of Spanish centres. 23,24 A cross-sectional study from the United States found that cardiac hospital admissions decreased by about 19 cases per week, with a rise in risk-adjusted mortality rates.12 Preliminary reports on stroke care described a 25% relative drop in admissions for stroke and a 14% drop in the use of thrombolysis among Italian stroke units in March 2020 compared with March 2019,8 with similar declines reported by a multicentre American study.25 A study of a stroke centre and provincial telestroke system in Ontario also found that fewer patents received preventative and acute stroke services in April 2020 than in previous years.26 We have shown not only that the early COVID-19 pandemic was associated with decline in presentations for ischemic stroke and use of acute therapies, even after adjustment for confounding variables, but that these problems persisted in later waves. Importantly, the lower population-level incidence of thrombolysis and endovascular therapy appeared to reflect declines in stroke presentations rather than any therapeutic inertia; the proportion of patients receiving thrombolysis was unchanged and the proportion receiving endovascular therapy increased in the second wave.
It is unlikely that the observed reductions in patients presenting to hospital with stroke reflect true declines in stroke occurrence, and more likely that it reflects pandemic-related hospital avoidance, as reported for other emergencies.27 Data from other jurisdictions have confirmed an association between SARS-CoV-2 positivity and subsequent cardiovascular events. For example, a self-controlled case series and matched cohort study from Sweden reported a nearly sevenfold increase in the odds of acute myocardial infarction and ischemic stroke in the 2 weeks after COVID-19.28 Therefore, especially during the second- and third-wave periods of our study, when COVID-19 cases surged, we expected to observe an increase in stroke presentations instead of declines. However, we noted a substantial increase in out-of-hospital deaths from stroke (as a proportion of all stroke-related deaths in the province) during the pandemic periods, lending some credence to our interpretation that more patients failed to present to acute care. This finding is consistent with reported increases in out-of-hospital cardiac arrests but must be interpreted cautiously because the classification of causes of death outside the hospital is challenging.29,30
Prehospital delays in time from onset of stroke to arrival at hospital, as observed in the lull period, confirm that patients with acute stroke in the early COVID-19 pandemic period tended to delay presenting to hospital, despite relatively low COVID-19 case numbers at the time. This observed delay is in keeping with previous small studies reporting delayed presentation for stroke and myocardial infarction.13,14 We did not find any increase in stroke severity among patients receiving acute stroke therapies in the pandemic periods we studied. One plausible explanation is that more severe strokes occurred among patients presenting to hospital who did not receive acute therapies (for whom we did not have severity data), given their higher odds of in-hospital death, as seen in Waves 2 and 3. The observation that minor strokes (i.e., patients discharged without hospital admission) were a smaller proportion of all ischemic strokes in the pandemic periods could imply that patients seeking medical attention had more severe strokes (as they were deemed to need admission). This might also reflect a greater reluctance among patients with minor strokes to seek care during the pandemic. The observed higher rates of out-of-hospital deaths from stroke also likely represent some patients with severe strokes who died before arrival at hospital during the pandemic. Regardless, our findings should motivate health care systems to ensure that recommendations to seek immediate care for conditions like stroke that require emergency treatment are not drowned out by pandemic-related messaging. It may be prudent for public health agencies to avoid blanket stay-at-home statements that may inadvertently promote hospital avoidance and ensure that statements promoting physical distancing or other measures are combined with messages encouraging people to still seek immediate attention for emergencies.
We observed fewer comorbidities among patients presenting with stroke in the early pandemic period compared to the prepandemic period. This might relate to early public health messaging during the pandemic, which warned patients with comorbidities that they were at higher risk of severe COVID-19, and may have promoted hospital avoidance.3 Patients with comorbodities may have instead contributed to out-of-hospital deaths from stroke. This skewed representation of comorbidities and other characteristics among patients presenting to hospital has been observed in other observational studies during the pandemic, and has been termed a “collider bias.”31 We saw some reversal of this trend in later waves, with patients more likely to have certain comorbidities than in the prepandemic period.
The observed in-hospital workflow delays (particularly for endovascular therapy) reflect the procedural changes necessary for pandemic care. Besides routine use of personal protective equipment, additional changes that occurred included enhanced cleaning protocols for the CT scanner and the angiography suite. Site-specific changes were also implemented. For example, the stroke team in Calgary was instructed to use a more infection prevention–compliant angiography suite that was several minutes away from the suite used before the pandemic. Preliminary data on such delays have come from different regions for acute coronary syndrome12,32 and stroke in the first wave,33 including a single-centre Canadian study that reported longer onset-to-door, door-to-needle and door-to-reperfusion times.34 A multicentre study of consecutive patients with stroke in the United States also reported a small but significant delay related to in-hospital workflow.35 Our data on recurrent delays in subsequent waves further underscore the need for ongoing vigilance by stroke teams with continuous re-evaluation of “code stroke” protocols to address inefficiencies, while adhering to precautionary measures. Importantly, our findings show that inefficiencies are not inevitable, with significantly shorter door-to-CT times (reflecting initial rapid triage and assessment by stroke teams) observed in Wave 2.2 than in the prepandemic period, and workflow times that essentially returned to the prepandemic baseline by Wave 3. No specific intervention was undertaken by the health care system to facilitate this, but ongoing feedback was provided throughout the province to achieve door-to-needle times shorter than 30 minutes, in addition to national guidance on adapting stroke workflows to the pandemic.36 It is also likely that Alberta’s emergency and stroke teams became more used to providing stroke care in the pandemic setting.
Limitations
We cannot know to what extent observed delays or declines were related to difficulties in accessing help rather than pure hospital avoidance. Because the cohort was defined by administrative data, some strokes may have been misclassified; however, our data sources and case definitions have been validated in previous audits.17 Workflow data were only available for patients who received acute therapies, likely resulting in an underestimation of delays, since patients presenting much later would be less likely to receive such therapies. Hospitals adopted additional infection prevention precautions, but we do not have specific data on why delays occurred in different settings. For example, several factors could have prolonged the time from groin puncture to reperfusion, such as if general anesthesia was used instead of conscious sedation or if care was potentially impeded by a heightened sense of caution and attention to the patient’s respiratory status.6,37 We did not have data on long-term outcomes to assess downstream effects of observed delays; however, poststroke outcomes are highly dependent on time to treatment.38 Our data set did not contain information on the COVID-19 status of patients, so assessing whether patients were managed differently based on their infectious state was not possible; however, all acute stroke patients were managed as potentially infectious in the first 24 hours, pending results of SARS-CoV-2 testing. Unmeasured covariates may have caused residual confounding in our adjusted analyses. We had only summary data on out-of-hospital deaths and therefore could not perform adjusted analyses of this measure.
Conclusion
In Alberta, the first year of the COVID-19 pandemic was associated with persistent population-level reductions in presentations and use of acute therapy for ischemic stroke, recurrent delays in stroke treatment and higher odds of in-hospital death from stroke in later waves. Public health messaging should encourage patients to seek care for emergencies and stroke systems should continuously re-evaluate “code stroke” protocols to mitigate inefficiencies.
Footnotes
Competing interests: Aravind Ganesh reports membership on the editorial boards of Neurology, Stroke, Frontiers in Neurology and Neurology: Clinical Practice; consulting fees from Atheneum, MD Analytics, MyMedicalPanel, Figure 1, CTC Communications, DeepBench, Research on Mind and Creative Research Designs; research support from Alberta Innovates, Campus Alberta Neuroscience, the University of Calgary, the Sunnybrook Research Institute INOVAIT program and the Canadian Institutes of Health Research (CIHR); honoraria from Figure 1 and Alexion; travel support from the American Academy of Neurology, the Association of Indian Neurologists in America, the American Heart Association and the University of Calgary; stock and stock options from SnapDx, TheRounds.com and Advanced Health Analytics (AHA Health); and a patent application (US 17/317,771) for a system for prehospital patient monitoring and assessment, and delivery of remote ischemic conditioning or other cuff-based therapies. Finlay McAlister holds the Alberta Health Services Chair in Cardiovascular Outcomes Research. Jessalyn Holodinsky reports funding from CIHR. Michael Hill reports patents (US Patent 62/086,077 and 10,916,346); stock in PureWeb and Circle Neurovascular; membership on the boards of the Canadian Federation of Neurological Sciences, the Canadian Stroke Consortium and Circle Neurovascular; grant support from Alberta Innovates Health Solutions, CIHR, the Heart & Stroke Foundation of Canada, the National Institutes of Neurological Disorders and Stroke, NoNO, Boehringer Ingelheim and Biogen; consulting fees from Sun Pharma; and participation on steering committees or data monitoring safety boards for the Oncovir Hiltonel trial, the DUMAS trial, BRAIN-AF trial, the ARTESIA trial, the OCEAN trial, CSPIN network, the PLAYSAFE trial, RACECAT trial and the SHINE trial. Eric Smith reports grant funding from the CIHR, Brain Canada and the Weston Brain Institute; consulting fees from Bayer, Biogen and Javelin; royalties from UpToDate; and payment from the American Heart Association for work as Associate Editor of Stroke. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Aravind Ganesh conceived the study, and Aravind Ganesh, Finlay McAlister, Oleksandr Shlakhter, Jessalyn Holodinsky, Balraj Mann, Michael Hill and Eric Smith contributed to the study design. Jilling Stang collected data, and Aravind Ganesh and Jillian Stang contributed to data analysis. Aravind Ganesh, Finlay McAlister, Michael Hill and Eric Smith interpreted the data. Aravind Ganesh wrote the first draft of the manuscript. All authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: The study was funded by the Canadian Cardiovascular Society through the COVID-19 Challenge for Canada Initiative.
Data sharing: All data used for this study are stored in a deidentified database and the programming code is stored as a STATA do-file. Requests for access to the data or code, accompanied by a proposal of appropriate rigour, will be considered by the corresponding author.
- Accepted February 1, 2022.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/