Abstract
Background: Although assessment of geriatric syndromes is increasingly encouraged in older adults, little evidence exists to support its systematic use by general practitioners (GPs). The aim of this study was to determine whether a systematic geriatric evaluation performed by GPs can prevent functional decline.
Methods: We conducted a controlled, open-label, pragmatic cluster-randomized trial in 42 general practices in Switzerland. Participating GPs were expected to enrol an average of 10 community-dwelling adults (aged ≥ 75 yr) who understood French, and had visited their GP at least twice in the previous year. The intervention consisted of yearly assessment by the GP of 8 geriatric syndromes with an associated tailored management plan according to assessment results, compared with routine care. Our primary outcomes were the proportion of patients who lost at least 1 instrumental activity of daily living (ADL) and the proportion who lost at least 1 basic ADL, over 2 years. Our secondary outcomes were quality-of-life scores, measured using the older adult module of the World Health Organization Quality of Life Instrument, and health care use.
Results: Forty-two GPs recruited 429 participants (63% women) with a mean age of 82.5 years (standard deviation 4.8 yr) at time of recruitment. Of these, we randomly assigned 217 participants to the intervention and 212 to the control arm. The proportion of patients who lost at least 1 instrumental ADL in the intervention and control arms during the course of the study was 43.6% and 47.6%, respectively (risk difference −4.0%, 95% confidence interval [CI] −14.9% to 6.7%, p = 0.5). The proportion of patients who lost at least 1 basic ADL was 12.4% in the intervention arm and 16.9% in the control arm (risk difference −5.1%, 95% CI −14.3% to 4.1%, p = 0.3).
Interpretation: A yearly geriatric evaluation with an associated management plan, conducted systematically in GP practices, does not significantly lessen functional decline among community-dwelling, older adult patients, compared with routine care.
Trial registration: ClinicalTrials.gov, NCT02618291.
The World Health Organization has defined healthy aging as the process of developing and maintaining functional ability that enables well-being in older age.1 Functional ability is often measured by an individual’s ability to perform activities of daily living (ADLs) without assistance. Geriatric syndromes, corresponding to multifactorial, chronic conditions, can impair physical and mental capacities,2–4 and are directly associated with functional decline.5 If recognized early, adapted preventive measures and management strategies can be started to limit functional decline.6–8 Interventions that have been shown to delay functional decline include comprehensive geriatric assessment, regular home visits and physical therapy.6,8,9 Comprehensive geriatric assessment consists of a “multidisciplinary diagnostic and treatment process that identifies medical, psychosocial, and functional capabilities of older adults to develop a coordinated plan to maximize overall health with ageing.”10 These assessments are usually performed by specialized geriatric teams for patients who have already been identified as frail or in the context of rehabilitation. However, most older patients see only their general practitioner (GP) and are not provided a comprehensive geriatric assessment, considering that this is a lengthy process that is often beyond the scope of a usual primary care consultation. A recent systematic review of comprehensive geriatric assessment in primary care found only 4 studies conducted in this setting,11 showing mixed effects on clinical outcomes. Only 1 study assessed functional ability, and it showed no impact in this context.12
In primary care, it may be more beneficial to use shorter screening tools.13–19 Previous studies using shorter tools adapted for primary care have failed to show a difference for patients compared with routine care.17,18 These interventions usually targeted patients who were already identified as frail or with a predefined number of problems.17,18,20
In contrast, our Active Geriatric Evaluation (AGE) tool targets all patients aged 75 and older. This clinical tool can be easily integrated to clinical encounters in GP practices, without the need for additional organizational changes. For this study, we aimed to determine whether the AGE tool, specifically designed for GPs and consisting of a brief assessment of the most relevant geriatric syndromes combined with management plans, could slow functional decline in older patients.
Methods
Study design
We conducted a controlled, open-label, pragmatic cluster-randomized trial in GP practices in western Switzerland. We used the PRECIS-2 criteria in designing the study to optimize direct applicability to GP practices. The study protocol is available at https://clinicaltrials.gov/ProvidedDocs/91/NCT02618291/Prot_001.pdf.
Participants
We planned to recruit at least 40 GPs (20 per arm), and each GP was expected to recruit 10 patients, on average. The GPs were recruited via letters sent by mail, professional societies’ newsletters or personal contact by email or telephone. Participating GPs had to work at least 20 hours per week as GPs in French-speaking Switzerland. Only 1 GP per practice could participate to limit contamination. We excluded specialists in geriatrics and GPs who had participated in the validation study of the AGE tool.
We included patients if they were at least 75 years old at recruitment, living at home, able to understand French and had visited their GP at least twice in the previous year. We excluded patients who had had a geriatric or specialized memory consultation in the 3 months before recruitment, or who were planning to leave the study area or change GP in the next 2 years. Patients were enrolled by GPs before GP group assignment was revealed. The GPs could choose between assessing every patient aged at least 75 years for study eligibility until the target number of inclusion was reached, or proposing the study only to a random sample of their patients. In this latter scenario, we asked GPs to decide on a fixed number of patients to be included per day (1 or 2), selected from the pre-eligible patients on the day’s agenda using a list of random numbers. Written consent was sought by GPs from individual participants before randomization.
Setting
Most Swiss GP practices are small, self-owned practices with 2–4 GPs. Integrated nurse practitioners and social workers are uncommon. Community-based services, such as home-based care, physical or occupational therapy are prescribed by GPs, but delivered outside of GP practices.
Randomization and blinding
The randomization unit was the GP, with GPs assigned on a 1:1 ratio to the intervention or usual care arm. An independent researcher generated a computer-based randomization list, using uneven block sizes. She then prepared sealed, opaque envelopes containing information on the treatment arm, with a printed identification number on the outside. During training sessions that took place after patient enrolment, GPs were assigned to their respective arm upon opening the envelope corresponding to their predefined unique identification number.
The research assistant performing the main outcome measures (telephone interviews), study coordinator and study statistician were blinded to the randomization. Participants, GPs and study assistants who conducted the annual visits to the family practice were unblinded to the GP’s assignment. Specific sections of the electronic case report form, which revealed assignment, were coded so that blinded staff could not link these data to participant or GP identifiers.
Intervention
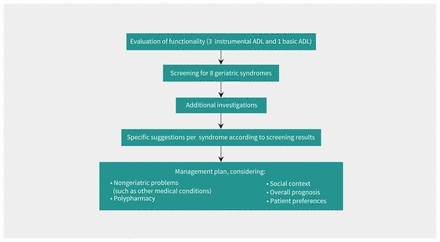
The intervention comprised a yearly, brief assessment, guided by the AGE tool (Figure 1) of 4 ADLs (3 instrumental ADL and 1 basic ADL) and screening for 8 geriatric syndromes, namely cognitive impairment, mood disorder, gait and balance impairment, visual impairment, hearing impairment, urinary incontinence, malnutrition and osteoporosis (Table 1), followed by proposal of a management plan based on the results of the evaluation. Details of the construction and validation of the AGE tool have been published elsewhere.15,21,22
Conceptual framework of the Active Geriatric Evaluation (AGE) tool. Note: ADL = activity of daily living. Adapted from Sen and Monod (licensed under CC BY 4.0).15
Active Geriatric Evaluation (AGE) tool
The suggestions for management were divided into 2 distinct steps: additional tests after a positive screen to confirm or exclude diagnoses, and specific management approaches. All suggested approaches were based on a literature review15 and geriatrician expertise. Management suggestions were further graded as major and minor. To preserve the pragmatic approach of the study, GPs were free to implement the proposed suggestions and approaches.
The AGE tool was available either as an electronic tool (embedded in the case report form) or as a paper summary. In the electronic form, sections of the management plan relative to specific geriatric syndromes appeared if selected in the screening process. In addition, GPs could add or delete specific sections of the management plan. We intended for the tool to be administered by GPs, but GPs could delegate the screening to medical assistants. General practitioners assigned to the intervention arm received a 2-hour, face-to-face, small group training session on the AGE tool from an academic GP and a geriatrician, and received a reference book on comprehensive geriatric assessment.23 The intervention was delivered during routine face-to-face consultations. If a single consultation was not sufficient, it could be spread over multiple consultations. Both GP groups received a 1-hour training session on study procedures. General practitioners of the control group received no specific training related to the study intervention.
General practitioners integrated follow-up patient assessments into regular consultations, with a final outcome visit encouraged after 2 years, plus a 3-month window. A research assistant conducted annual reviews of medical records in the practice, extracting data on the number of consultations and content (e.g., type of clinical examination, weight and height measures, evaluations of alcohol consumption, nutrition and physicial exercise), laboratory tests, radiological evaluations, new diagnoses of chronic conditions (coded using the International Classification of Primary Care, version 2), medications, specialist referrals, emergency consultations and hospital admissions. In parallel, a different research assistant conducted annual telephone interviews to assess patient-reported outcomes.
Outcomes
The primary outcomes were the proportion of patients who lost independence in at least 1 instrumental ADL and the proportion of patients who lost independence in at least 1 basic ADL over 2 years. We scored ADLs as 0 or 1,21 considering 8 instrumental ADLs (i.e., using the telephone, shopping, food preparation, housekeeping, laundry, mode of transportation, responsibility for own medications and ability to handle financing)24 and 6 basic ADLs (i.e., bathing, dressing, toileting, transferring, continence and feeding).25 These 2 measures have been used in numerous studies because of their robust psychometric properties, their sensitivity to change, their simplicity, and the fact that they can be reliably evaluated over the telephone.26,27 According to previous studies that used this outcome in similar populations, avoiding a 1-point (i.e., 1 activity) loss out of 8 activities (for instrumental ADLs) can be considered clinically meaningful.8,28 We decided against treating the outcome as a continuous variable (and comparing the mean difference between the 2 arms) after preliminary analysis of baseline data showed that most patients had a baseline instrumental ADL score of 8 (maximal score).
Secondary outcomes included mean quality-of-life score, measured using the older adult module of the World Health Organization Quality of Life Instrument (WHOQOL-OLD), and incidence of hospital admissions, institutionalizations, emergency department visits and outpatient visits. We also compared the 2 groups in terms of the number of geriatric syndromes identified and the adopted management strategies, such as medication adaptation, referral to specialty care or supportive measures.
We asked GPs to record any serious adverse events (e.g., hospitalization, death) in the electronic case report form within 7 days of their occurrence. For each serious adverse event, GPs collected the time of onset, duration, resolution, action to be taken, assessment of intensity and relation to the study intervention. For every step of the management plan, GPs recorded whether they adhered to the suggestion or not. We estimated GP adherence to the intervention at each step of the AGE tool. In the study protocol, we prespecified quantitative outcomes of acceptability (i.e., proportion of GPs using the tool once or twice during the study follow-up) and feasibility (i.e., physician adherence to management sugggestions). We evaluated these outcomes in combination with our qualitative assessment of acceptability and feasibility, as well as perceptions of autonomy, assessed by semistructured interviews of a subset of patients and GPs. The results from the qualitative component of this research have been presented elsewhere.29
Statistical analysis
To estimate the sample size, we assumed that 10% of patients would lose independence in at least 1 instrumental ADL in the intervention arm and 25% in the control arm. These proportions were based on previous trials6–8 and longitudinal studies. 3 Using these parameters, we generated cluster data with various combinations of the number of GPs per arm and patients per GP for different levels of intraclass correlation coefficient (ICC). To achieve a power of 90%, 8 patients per GP would provide a sufficient sample size if we had 20 GPs per arm, based on an ICC of 0.10. Assuming a loss to follow-up of 15%, we increased the number of patients per GP to 8/(1–0.15) = 10, corresponding to a final sample size of 40 GPs with a total of 400 patients.
For our primary analysis, we compared the proportions of patients in the intervention and control groups who had lost at least 1 instrumental ADL and those who had lost at least 1 basic ADL after 2 years, using a generalized logistic mixed-effect model, including a random effect for the physician.
For our secondary analyses, prespecified in the statistical analysis plan, we compared the mean reduction in WHOQOL-OLD score after 2 years between the intervention and control arms using a generalized linear mixed-effect model. We also compared the proportions of patients with hospital admissions, institutionalizations and emergency department visits by arm, as well as the number of routine visits, and the time to institutionalization or death. All comparisons between treatment arms used mixed models that included a random effect for cluster. We used mixed-effect negative binomial regression to compare the number of GP consultations, specialist consultations and patient weight measurements, and mixed-effect logistic regression for binary secondary outcomes (i.e., patients with at least 1 emergency consultation, hospital admission, stay in an institution, new chronic condition diagnosis, severe adverse event, communication between GP and home-based care, communication between GP and the family and presence of polymedication). We adopted Cox survival analysis techniques to compare time to institutionalization or death. We used longitudinal models, including a second random effect for a participant’s repeated measures, to estimate the 2-year evolution of the mean number of instrumental ADLs and basic ADLs (2-level, mixed-effect Poisson models) and mean WHOQOL-OLD scores (2-level, mixed-effect linear model).
All patients recruited by the randomized GPs were included in the analysis. The primary analysis was a complete case analysis that included all patients with instrumental or basic ADLs measured at baseline and after 2 years. After complete case analysis, we performed prespecified sensitivity analyses with the last observation carried forward, and considering patients who had died or who were institutionalized as having lost 1 instrumental ADL (full analysis set). We performed an additional sensitivity analysis that included all patients with baseline outcome assessment, where all those missing follow-up assessment were considered as having lost 1 instrumental or basic ADL. We also analyzed the per-protocol population, excluding participants in the intervention arm who received fewer than 2 complete screenings (i.e., at least 7 out of 8 items screened) or participants for whom GPs followed fewer than half of the proposed major suggestions.
We conducted analyses in R version 3.5, and Stata version 16.1. The steering committee, composed of all investigators, reviewed the planned interim analysis of primary and secondary outcomes when at least 50% of patients had their 1-year assessment, and reviewed the safety analysis on serious adverse events.
Ethics approval
This study was approved by the cantonal ethics committee on May 30, 2016 (CER-VD no. 2016-00422). The study followed the World Medical Association’s Declaration of Helsinki. Participants gave written informed consent before taking part.
Results
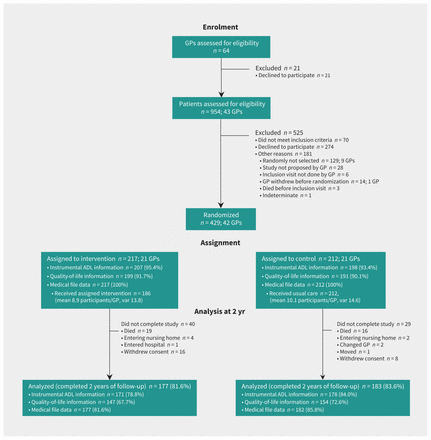
We recruited 42 GPs to participate in the study. Of 954 patients screened for eligibility between Aug. 12, 2016, and Nov. 15, 2017, GPs recruited 429 patients between Sept. 26, 2016, and Jan. 29, 2018 (Figure 2), with follow-ups completed by Jan. 31, 2020. Among participating patients, 63% were female and the mean age was 82.5 years (standard deviation 4.8 yr). Baseline sociodemographic and clinical characteristics are shown in Table 2.
Study flowchart. Note: ADL = activity of daily living, GP = general practitioner, var = variance.
Baseline patient characteristics
In the primary analysis, the proportion of patients who lost independence in at least 1 instrumental ADL during the course of the study was 43.6% and 47.7% in the intervention and control arms, respectively (risk difference −4.0%, 95% confidence interval [CI] −14.9% to 6.7%, p = 0.5) (Table 3). The proportion of patients who lost independence in at least 1 basic ADL was 12.4% and 16.9% in the intervention and control arms, respectively (risk difference −5.1%, 95% CI −14.3% to 4.1%, p = 0.3).
Proportion of patients who lost activities of daily living (ADLs) between baseline and 2 years assessment, according to treatment arm
The mean reduction in quality-of-life scores in the intervention and control groups was −0.12 (SD 7.36) and 0.74 (SD 7.76), respectively (p = 0.3). We did not observe significant differences between study arms for any secondary outcomes assessed regarding health care use (i.e., number of consultations, emergency consultations, hospital admissions or institutional stays), type of health care (i.e., number of weight measures and number of specialists involved) or communication with home-based care or families (Table 4). Time to institutionalization or death was not different between patients receiving AGE or usual care by log-rank test (p = 0.27). The proportion of patients experiencing severe adverse events was not different between the treatment arms.
Secondary and safety outcomes, according to treatment arm
In the sensitivity analysis, considering death or admission to an institution as having lost at least 1 instrumental ADL increased the number of patients analyzed in the complete case analysis from 339 to 381. We did not observe a significant difference between arms in the proportion of patients who had lost at least 1 instrumental ADL (50.0% in the intervention and 51.8% in the control arms, respectively, p = 0.7, ICC = 0.00) (Appendix 1, supplementary material, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.202887/tab-related-content). Longitudinal analysis of instrumental ADL and basic ADL scores for the entire study population, and stratified by age and gender, can be found in Appendix 1.
Overall, of 179 patients with a baseline screening using the AGE tool, 119 (66.5%) were screened again after 1 year. A median of 3 (interquartile range [IQR] 1 to 4) syndromes was suspected at the first visit, and a median of 2 (IQR 1 to 3) syndromes was suspected at the second visit. Overall, GPs adhered to 59.9% of all the major suggestions of the management plan. In the intervention group, GPs adhered to at least 50% of the suggested items for 48.2% of patients (details provided in the supplementary material, Appendix 1). Overall adherence, including both the assessment and management plan, was 61.6% (95% CI 48.5% to 74.7%, adjusted for cluster). In the per-protocol population, which excluded 118 patients in the intervention arm with low GP adherence, we did not observe any significant differences between the intervention and control arms in the proportion of patients who had lost at least 1 instrumental ADL (45.7% v. 47.6%, p = 0.8, ICC = 0.01), in the proportion of patients who had lost at least 1 basic ADL (10.9% v. 16.6%, p = 0.3, ICC = 0.10) or in the WHOQOL-OLD score after 2 years (−0.32 v. 0.71, p = 0.4, ICC = 0.01) (Appendix 1).
Interpretation
Systematic screening for and management of geriatric syndromes in general practice using the AGE tool (which screens for most of the items used in similar tools30 and covers all areas recommended by WHO’s approach to integrated care for older people [ICOPE]19) did not slow functional decline of patients aged 75 years and older over a 2-year course compared with routine care. The intervention also showed no effect on quality-of-life scores or health care use. In our study, the intervention was a brief assessment delivered by GPs, rather than a comprehensive geriatric assessment delivered by geriatricians or specifically trained nurse practitioners, as is often used in other trials. Our results differ from those of studies that used home-based management programs, which have shown a positive effect in preventing functional decline in participants.8,9,31 Other studies using shorter tools adapted for GPs have also failed to show an impact on functional decline.17,18 These interventions targeted patients identified as frail or with a predefined number of problems, whereas our study included all patients aged 75 and older. We observed higher functional decline than anticipated in our study participants, as well as a higher-than-anticipated proportion of deaths, which, we think, highlights the importance of targeting all older adults with such interventions.
Several reasons may explain the absence of effect of the intervention. First, unlike trials targeting underserved populations, 31 usual care in the Swiss context may already be very good, as shown by the high proportion of patients already equipped with hearing aids or having undergone cataract surgery at baseline. Second, the intervention was of moderate intensity and pragmatic nature, which may have lessened its impact. A more controlled implementation of all items of the management plan may have allowed us to detect a difference in patient outcomes. However, the fact that we did not observe a difference in the per-protocol population does not support the hypothesis of insufficient adhesion. Also, as further investigations or interventions were negotiated with patients in the long term, they may not have been captured within the trial’s timeframe.
Although a substantial body of evidence describes the processes and predictors of functional decline, few interventions have successfully modified individual life-course trajectories. The question of when and in what population to begin interventions is still unresolved. Evidence from the Whitehall II study suggests that prevention of frailty should begin in midlife,32 but how and when to promote this patient–provider dialogue should be the objective of future research.
Despite our negative findings, we would argue that GP practices are the right setting to guide patients in aging because of their wide population coverage. However, interprofessional teams, rather than physicians alone, may better deliver this care, which should also include assessment of patients’ goals. In Switzerland, most physicans in primary care work alone and rarely in multprofessional teams;33 however, other jurisdictions, such as the United Kingdom, the Netherlands or Canada, are more familiar with integrated practices, with physicians, nurses and other health care professionnals working together. It is likely that such settings, with stronger interprofessional collaborations and better organization of care delivery at the system level, may allow for consolidated patient management strategies and better functional outcomes, even though GPs continue to play a key role in multidisciplinary teams.34,35
Limitations
One area of concern is bias due to deviation from the intended intervention. Indeed, GPs in the control arm may have been more attentive to the functional issues of their older patients because of their participation in the study. Furthermore, GPs in the intervention arm did not adhere to all suggestions of the tool, which was consistent with the pragmatic nature of the trial. In terms of external validity, a certain amount of selection is unavoidable when conducting trials that involve a substantial investment from participating GPs. Participating GPs may have been more interested in geriatric care and be more up-to-date with continuing education in this area. Thus, practices in the control arm may have provided better care than average practices. In addition, GP practices in Switzerland are very physician-centred. Our study results may not be valid in different primary care settings where interprofessional teams could have enhanced the impact of the intervention.
Our patient recruitment method may have favoured slightly younger patients (Appendix 1, Table S1) and may have excluded the most vulnerable patients. Apart from this, we did not observe important baseline differences between patients in the intervention and control groups. Loss to follow-up was within the expected range and comparable between groups. The main area for concern was missingness of certain outcomes, such as ADL or quality-of-life scores, where missingness was potentially dependent on the score value. However, the sensitivity analysis in which we considered missing outcomes as negative is reassuring on this point. Finally, our assumptions regarding loss of independence that guided the sample size estimation were overly optimistic. We did not adjust expected rate of functional decline or p values to the fact that we had 2 primary outcomes; we calculated sample sizes using only expected rates of instrumental ADL loss, not basic ADL loss. As our findings were negative, however, this does not affect the general thrust in the results.
Our choice of outcomes could be criticized. First, the use of a disability criterion in instrumental ADLs causes a number of methodological problems (i.e., choice of items and categories, ceiling effect, lack of gender sensitivity).36 Second, many study participants found the WHOQOL-OLD to be intrusive, resulting in some participant withdrawals or incomplete data. Few clinical chronic care interventions have actually been able to improve patient quality of life.37 We did not include patient-centred measures, such as measures of patient satisfaction or continuity of care,38–40 as our intervention did not target patient-centredness or integrated care per se. The AGE intervention did not explicitly elicit patient goals. More recent approaches that promote goaloriented care and assess individual goal attainment are promising. 41–43 On the other hand, studies in the field of multimorbidity have shown contrasting results on quality of life, even if a goal-oriented, patient-centred approach was used.37
Conclusion
Encouraging GPs to screen older patients for geriatric syndromes using a brief assessment of activities of daily living and to propose management approaches is not sufficient to slow the functional decline of patients aged 75 and older. System-level interventions may be needed to promote integrated care that includes patient preferences.
Acknowledgements
The authors thank all participating general practitioners (GPs) and patients, and all geriatricians who reviewed the AGE recommendations and participated in GP training. The authors also thank Dr. Ophélie Viret for her contribution to study implementation and acquisition of the data, Prof. Jacques Cornuz and Prof. Christophe Büla for their input into the study conception. The authors thank the GPs of the pilot committee for guiding the case report forms and study procedures, and the study staff for data collection and monitoring. The authors thank Melanie Price Hirt for English language editing.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Stéfanie Monod and Nicolas Senn conceived the intervention. Yolanda Mueller, Isabella Locatelli and Nicolas Senn designed the study. Yolanda Mueller and Nicolas Senn supervised data collection, and Yolanda Mueller, Joëlle Schwarz and Isabella Locatelli analyzed the data. All authors contributed to data interpretation. Yolanda Mueller wrote the first draft of the manuscript. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: The trial was funded by the Swiss National Science Foundation Grant 32003B_159863/1 until February 2019. Trial completion was self-funded.
Data sharing: The supporting data for this article are available on request on the Unisanté data repository: https://doi.org/10.16909/dataset/23.
Disclaimer: The funder had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. All authors had full access to all of the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
- Accepted June 1, 2021.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/