Abstract
BACKGROUND: The emergence of cell-free fetal DNA (cfDNA) testing technology has disrupted the landscape of prenatal screening for trisomies 21 (T21) and 18 (T18). Publicly funded systems around the world are grappling with how to best integrate this more accurate but costly technology, as there is limited evidence about its incremental value in real-world conditions. The objectives of this study were to describe the population-based performance of Ontario’s prenatal screening program, which incorporates publicly funded cfDNA screening for specific indications, and the effect of cfDNA testing on the screening and diagnostic choices made by pregnant people.
METHODS: We conducted a retrospective, descriptive cohort study using routinely collected data from Better Outcomes & Registry Network (BORN) Ontario, which captures linked population data for prenatal and neonatal health encounters across Ontario. We included all singleton pregnancies with an estimated due date between Sept. 1, 2016, and Mar. 31, 2019, that underwent publicly funded prenatal screening in Ontario, and a comparison cohort from Apr. 1, 2012, and Mar. 31, 2013. We assessed performance of the screening program for the detection of T21 or T18 by calculating sensitivity, specificity, positive predictive value and negative predictive value against diagnostic cytogenetic results or birth outcomes. We assessed the impact of the program by calculating the proportion of T21 screen-positive pregnancies undergoing subsequent cfDNA screening and invasive prenatal diagnostic testing.
RESULTS: The study cohort included 373 682 pregnancies. The prenatal screening program had an uptake of 69.9%, a screen-positive rate and sensitivity of 1.6% and 89.9% for T21, and 0.2% and 80.5% for T18, respectively. The test failure rate for cfDNA screening was 2.2%. Invasive prenatal diagnostic testing decreased from 4.4% in 2012–2013 to 2.4% over the study period; 65.2% of pregnant people who received a screen-positive result from cfDNA testing went on to have invasive prenatal diagnostic testing.
INTERPRETATION: This publicly funded screening program, incorporating cfDNA analysis for common aneuploidies, showed robust performance, a substantial reduction in invasive prenatal diagnostic testing and that pregnant people exercise autonomy in their choices about prenatal screening and diagnosis.
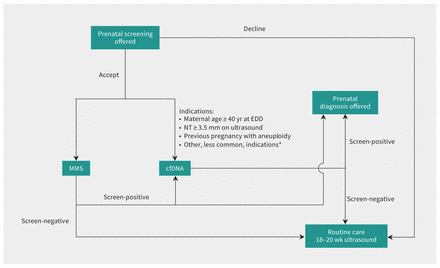
Offering screening for chromosomal aneuploidies, including trisomies 21 (T21) and 18 (T18), is part of routine prenatal care. To this end, multiple marker screening, which uses a combination of ultrasound and maternal serum biomarkers, has been publicly funded in Ontario since the 1990s (Appendix 1, Table S1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.202456/tab-related-content).1 In 2012, private diagnostic laboratories began marketing cell-free fetal DNA (cfDNA) testing across Canada. Also known as noninvasive prenatal testing, cfDNA analysis is a maternal blood test that analyzes fetal DNA that originates in the placenta. This disruptive technology shows better detection of fetal aneuploidies with fewer false-positive results than multiple marker screening,2 and its adoption has led to fewer invasive procedures for prenatal diagnosis.3,4 Market forces and concerns over equitable access led to rapid and variable adoption of cfDNA analysis into prenatal screening programs across Canada and internationally.4–7 The current price (about Can$390)8 of cfDNA analysis precludes it from being publicly funded as a universal test. Instead, publicly funded programs often use a contingent model, offering cfDNA screening for pregnancies with specific indicators that increase the likelihood of aneuploidy.9 However, indications for accessing cfDNA screening vary widely across jurisdictions.10,11 Since 2016, publicly funded cfDNA screening has been offered in Ontario for the following specific indications: a screen-positive result from multiple marker screening, maternal age > 40 years at estimated due date, certain concerning findings on fetal ultrasound or a previous pregnancy with aneuploidy (Figure 1).
Ontario’s universal and publicly funded model for incorporating cfDNA analysis into prenatal screening for aneuploidy. In 2016, Ontario began funding cfDNA prenatal screening for the common autosomal aneuploidies (e.g., trisomy 21, 18). Pregnant patients at high risk for fetal aneuploidy are eligible for publicly funded cfDNA screening after a screen-positive MMS (defined as risk ≥ 1 in 350 for the enhanced first trimester screening test or risk ≥ 1 in 200 for the quadruple marker screening test) or as a first-tier screen. Criteria for determining eligibility for first-tier cfDNA screening are based on recommendations from the provincial advisory group, with the goal of optimizing performance and containing costs.12 Although presented as a flowchart, this figure does not represent chronological care pathways or a stepwise approach; in the real world, decisions about screening and testing do not always follow a model. Note: cfDNA = cell-free fetal DNA screening, EDD = estimated due date, MMS = multiple marker screening, PND = prenatal diagnostic testing, NT = nuchal translucency. *See Prenatal Screening Ontario’s website for all indications for first-tier cfDNA screening (https://prenatalscreeningontario.ca/en/pso/about-prenatal-screening/nipt-funding-criteria.aspx).
Prenatal Screening Ontario (www.prenatalscreeningontario.ca) is a government-funded program that coordinates provincial prenatal screening, facilitates the incorporation of evolving technologies and screening options, and is responsible for ongoing quality assurance reporting. Their policy planning is informed by data from Ontario’s perinatal registry, Better Outcomes Registry & Network (BORN) Ontario (www.bornontario.ca) (Appendix 1, Supplemental Figure S1). Evaluating the effect of new technologies (e.g., test accuracy, downstream testing, patient choice) is critical to screening programs13 and depends on rigorously collected and analyzed data. Previously published analyses from other multiple marker or contingent cfDNA screening programs are limited by a lack of population-level data linking prenatal screening results with cytogenetic records or birth outcomes.3,14–17
Our primary objective for this study was to report on the overall and modality-specific performance of Ontario’s prenatal screening program, wherein the offer of cfDNA screening is contingent on specific criteria that increase the likelihood of T21 or T18 (Figure 1). Our secondary objective was to report on the impact of this screening approach on test utilization by pregnant individuals.
Methods
Study design and data source
We conducted a retrospective, population-based, descriptive cohort study that used routinely collected data within BORN. Established in 2009, BORN is a prescribed registry under the Personal Health Information Protection Act18 that collects critical health data about every pregnancy, birth and newborn in Ontario directly from fertility clinics, screening and diagnostic laboratories, hospitals, midwifery practice groups and other organizations across the province.19 All publicly funded multiple marker and cfDNA screening is performed by Ontario-based laboratories that contribute complete testing data to BORN. This registry currently holds data for more than 1.4 million mother–newborn dyads, including linkable population data on all prenatal screening modalities, pre- and postnatal cytogenetic results, and birth outcomes (i.e., born live or deceased, suspected or confirmed congenital anomalies).20 Details about BORN’s data quality, availability, cleaning and linkage have been previously described.20
Inclusion and exclusion criteria
We included all singleton pregnancies in Ontario with an estimated due date between Sept. 1, 2016, and Mar. 31, 2019, that underwent publicly funded prenatal screening. We excluded records from self-funded cfDNA screening as these fall outside the scope of our public screening system. We deterministically linked patient postal codes to Census data to obtain neighbourhood income quintiles.
We also included a cohort of singleton pregnancies with an estimated due date between Apr. 1, 2012, and Mar. 31, 2013, to evaluate the effect of cfDNA screening on uptake of prenatal diagnostic testing. We chose this timeframe as cfDNA screening became sporadically available in the latter part of 2013.
Outcome measures and construction of cohorts
To meet our primary objective, we calculated the sensitivity (detection rate), specificity, and screen-positive rate of Ontario’s prenatal screening system for T21 and T18. A challenge for ascertaining the T21 and T18 status of all pregnancies that underwent prenatal screening is that diagnosis of aneuploidy via cytogenetic testing is performed only for a small subset of pregnancies and infants with appropriate clinical indications. Thus, we used BORN registry data for pregnancies without diagnostic results. Infants (and linked pregnancies) were presumed unaffected when birth records showed that neither T21 nor T18 were identified by, at minimum, 3 months of age. To form binary classification tables, we constructed 6 performance analysis cohorts from this data set to evaluate overall and modality-specific (multiple marker or cfDNA) screening for either T18 or T21. We excluded records from performance analysis cohorts for any of the following reasons: a screening record with no result; a cytogenetic result designated as mosaic, partial, uninterpretable or inconclusive; or a record with no associated abnormal or normal cytogenetic outcome and no negative birth outcome. Exclusion criteria were not mutually exclusive.
To meet our secondary objective we calculated the uptake of prenatal screening for T21, the number of pregnancies that were screen-positive using multiple marker screening that underwent either cfDNA screening or invasive prenatal diagnostic testing, and the number of pregnancies that were screen-positive using cfDNA screening that underwent prenatal diagnostic testing. We also compared the rate of screened pregnancies that underwent prenatal diagnostic testing of our main cohort with our 2012–2013 cohort, expressed as a proportion of all screened pregnancies in Ontario.
Statistical analyses
We summarized demographic data using means and standard deviations or counts and percentages, where appropriate. We calculated Clopper–Pearson confidence intervals for the performance measures. We extracted data from the source database using SAS software version 9.4 and prepared them for analysis and analyzed using R version 3.5.2.
Ethics approval
This study was approved by the research ethics boards of Children’s Hospital of Eastern Ontario (protocol 19/06PE), Ottawa Health Sciences Network (protocol 20190482-01H) and Mount Sinai Hospital (protocol 19-0181-C).
Results
Participants and demographics
Over the study period, we analyzed 373 682 eligible singleton pregnancies with access to a variety of screening modalities (Appendix 1, Supplemental Figure S2). Pregnant people who had first-tier cfDNA screening were older (as expected as per eligibility criteria) and had a higher income than those those who had either multiple marker screening alone or multiple marker and cfDNA screening. Table 1 describes maternal characteristics, and stratifies the number of pregnancies by screening modality.
Maternal demographics of singleton pregnancies in Ontario with an estimated due date between Sept. 1, 2016, and Mar. 31, 2019, excluding those with a self-paid screening test
Screening performance
Prenatal screening for either T21 or T18 was performed in 261 096 (69.9%) of singleton pregnancies. Not every pregnancy had a screening result returned for both T21 and T18; record inclusion and exclusion for each performance analysis cohort is illustrated in Figure 2. Of all screened pregnancies, 97.8% had multiple marker screening, of which 96.1% were included in our performance analysis for both T21 and T18. Of 22 558 cfDNA screening records, 93.6% were included in our analysis for both T21 and T18. The overall analyses of all 261 096 pregnancies with either multiple marker or cfDNA screening included 250 594 (96.0%) records for T21 and 250 600 (96.0%) for T18.
Construction of performance analysis cohorts. We applied eligibility criteria to all singleton pregnancies between Sept. 1, 2016, and Mar. 31, 2019, to construct 6 cohorts for analysis of overall and modality-specific (multiple marker or cfDNA) screening for either T18 or T21. Reasons for exclusion were not mutually exclusive and individual pregnancies may be represented in multiple cohorts. Note: cfDNA: cell-free fetal DNA, cyto = cytogenetic testing, MMS = multiple marker screening, T18 = trisomy 18, T21 = trisomy 21. *Ineligible cytogenetic results were those designated as mosaic, partial, uninterpretable or inconclusive. †Small numbers (n < 6) were suppressed.
The overall sensitivity (detection rate) of our contingent prenatal screening program was 89.94% for T21 and 80.47% for T18 (Table 2). Of all pregnancies that had been screened, 4216 (1.6%) had screen-positive results for T21 and 639 (0.2%) had screen-positive results for T18, where cfDNA screening results are considered definitive. Overall system specificity was 98.76% for T21 and 99.89% for T18, with a negative predictive value of > 99.9% for both T21 and T18. Given privacy restrictions inherent with the use of registry data, we are unable to provide counts when the cell sizes are fewer than 6, and therefore cannot report the number of false-negative results.
Overall and modality-specific performance of a universal and publicly funded prenatal screening program for trisomies 21 (T21) and 18 (T18)
Of all pregnancies that had been screened, 14 091 (5.4%) pregnancies had an unknown outcome (i.e., they lacked both a follow-up cytogenetic test result and birth outcome) (Figure 2), of which 668 (4.7%) were screen-positive for T21.
Impact of cfDNA screening on follow-up testing
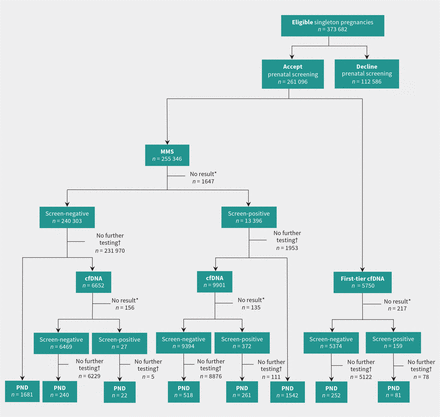
We focused on T21 when evaluating the different prenatal screening and diagnostic testing options pursued after a screen-positive result (Figure 3). For 13 396 pregnancies with a screen-positive result from multiple marker screening, 1953 (14.6%) had no further testing and 11 443 (85.4%) underwent follow-up testing. Of the latter, 9901 (86.5%) had cfDNA screening and 1542 (13.5%) went directly to prenatal diagnostic testing. Of the 372 pregnancies that were screen-positive after both cfDNA and multiple marker screening, 261 (70.2%) had invasive prenatal diagnostic testing. Of 159 pregnancies with a positive result after first-tier cfDNA screening, 81 (50.9%) had prenatal diagnostic testing. Overall uptake of prenatal diagnostic testing after a cfDNA screen-positive result was 65.2%.
Uptake of screening and invasive prenatal diagnostic testing (PND) for trisomy 21 via Ontario’s prenatal screening program. Note: cfDNA = cell-free fetal DNA screening; MMS = multiple marker screening. During the 2.5-year study period, an offer of prenatal screening was accepted for 261 096 singleton pregnancies (69.9%). This flowchart illustrates the variety of screening and testing options pursued by pregnant people in Ontario. Real-world utilization is very different than the ideal model presented in Figure 1. *“No result” refers to test failures and includes multiple test attempts. †“No further testing” refers to no further publicly funded cfDNA or invasive testing for aneuploidy.
We observed a 45% reduction in invasive prenatal diagnostic testing since the integration of cfDNA screening; 6242 (2.4%) of all 261 096 screened pregnancies in our main cohort underwent prenatal diagnostic testing, compared with 4208 (4.4%) of 96 501 screened pregnancies in the 2012–2013 cohort that predates the introduction of cfDNA screening.
Interpretation
Our study provides a large, population-based performance analysis of a contingent cfDNA prenatal screening system, leveraging BORN’s perinatal data set.20 From a population of 373 682 pregnancies, we report an overall uptake of 69.9%, a screen-positive rate and sensitivity of 1.6% and 89.9% for T21, and 0.2% and 80.5% for T18, respectively. In agreement with other publications, 21,22 our data show a cfDNA screening sensitivity of 99.8% for T21 and 94.4% for T18, with a test failure rate of 2.2% (including multiple attempts). Importantly, we observed a twofold reduction (56%) in invasive prenatal diagnostic testing since the integration of cfDNA screening, which is consistent with other publications. 3,4,23 Ontario’s prenatal screening system was designed based on modelled scenarios12 and was intended to align with optimal population-based screening principles;13 namely, to provide equitable access to cfDNA tests, to optimize detection and to reduce the overall screen-positive rate without substantially increasing costs. A 2014 cost and performance analysis of 8 modelled screening scenarios showed that integrating cfDNA as a contingent test improved the detection of T21 and reduced prenatal diagnostic testing with a modest increase in costs.12 Compared with this modelled scenario,12 we observed a slight increase in uptake (70% v. 67%) and similar performance.
Our study extends previous research by offering a fully linked, population-based performance assessment of a contingent cfDNA prenatal screening system in Ontario, Canada’s most populous province. A key strength of our approach was using linked BORN data to determine the T21 and T18 status of all pregnancies undergoing prenatal screening, which allowed us to identify true negative cases and report on specificity and negative predictive value. A 2018 study from the Danish Fetal Medicine Database reported the performance of a prenatal screening system with contingent cfDNA analysis similar to Ontario’s, but the Danish cohort was hospital based, far smaller (n = 6449) and more homogeneous. 24 Compared with the Danish system, which uses lower risk cutoffs for multiple marker screening25 to achieve its superior performance (100% sensitivity with 98.8% specificity),24 we show that a quality screening system with high performance is still attainable with more stringent cutoffs for multiple marker screening. Determining appropriate cutoffs is important in a public system, both to control costs and to avoid potential harms arising from false-positive results;9,26 Prenatal Screening Ontario is actively exploring how to optimize this aspect of our system.9,26
Routinely collected population data allow us to track the use of different tests as pregnant people navigate the program. Pregnant people may not follow a standard care pathway; some have cfDNA screening before or at the same time as multiple marker screening. Indications prompting cfDNA screening or prenatal diagnostic testing may occur in spite of a negative result from multiple marker screening, most commonly maternal age > 40 years or a subsequent ultrasound finding, thus accounting for the uptake of cfDNA screening and prenatal diagnostic testing even with a negative result from multiple marker screening.
The high accuracy and noninvasive nature of cfDNA screening has led to general concerns regarding the routinization of this test and its effect on informed choice.27,28 Ontario’s pregnant population pursued a variety of options after a screen-positive result, consistent with other publications23 showing that pregnant people make individual choices. Notably, we observed that although there is considerable uptake of publicly funded cfDNA screening, only 65.2% of people who received a positive result from cfDNA screening (contingent or first-tier) went on to have invasive prenatal diagnostic testing. Professional organizations uniformly advise diagnostic confirmation via cytogenetic testing before interrupting a pregnancy;29 therefore, our data may be interpreted to show that pregnant people are supported in making informed choices, including pregnancy continuation. The high positive predictive value of cfDNA screening allows for informed care to provide the best prenatal and perinatal outcomes after a screen-positive result from cfDNA screening. We observed an almost twofold (45%) overall reduction in invasive prenatal diagnostic testing since the integration of cfDNA screening, almost certainly because of its very low false-positive rate.
Given the sample size and data quality, our study provides insights for other jurisdictions, and shows that integrating cfDNA screening as a contingent (rather than universal) test can yield excellent performance and reduce unnecessary prenatal diagnostic testing for T21 and T18. We also show the value of comprehensive, registry-based data to monitor and optimize system performance. Using these findings as a baseline and with the power of registry data, Prenatal Screening Ontario can determine the cost-effectiveness of different screening models and algorithms. These data can also rapidly inform evidence-based guidance regarding the integration of new technologies (e.g., new approaches to cfDNA screening for aneuploidy), the detection of other conditions (e.g., microdeletion syndromes, preeclampsia, congenital anomalies) and adjustments for changing conditions (e.g., the impact of COVID-19 restrictions on access to ultrasound for nuchal translucency measurement).
Limitations
Despite our comprehensive data set, 5.4% of screened pregnancies were excluded from performance analyses because they had an unknown outcome; this is an inherent limitation of retrospective cohort studies. The proportion of unknown outcomes is similar to the predicted spontaneous pregnancy loss after 12 weeks of about 6%.30,31 Not unexpectedly, we observed substantially more screen-positive results among people with an unknown outcome compared with those with a known outcome (4.7% v. 1.6%) as pregnancy loss is associated with up to a 70% chance of aneuploidy;32–34 the exclusion of these records likely attenuates the reported performance.
Conclusion
Previous performance estimates for Ontario’s prenatal screening system were based on modelled data. Using robust linkage of prenatal screening results with birth outcomes, including all true negatives, we show that our real system achieves higher uptake and better detection rates than our previous modelling predicted. We also observed a twofold reduction in invasive prenatal diagnostic testing since the integration of cfDNA screening. We illustrated the power of registry data to monitor real-world performance of prenatal screening systems and enable real-time optimization; these data are used to drive continuous quality improvement of our program and to answer important questions about the use and utility of emerging screening methods.
Acknowledgements
The authors acknowledge the more than 370 000 pregnant people whose prenatal screening experience is reflected in this report, the various Ontario-based laboratories whose data were essential to this work, the birthing hospitals and midwifery practice groups who provide Ontario’s birth data and the team at BORN Ontario who ensure that these data are complete and accurate. The authors also thank Mari Teitelbaum for her contribution to the creation of the Prenatal Screening Ontario program.
Footnotes
Competing interests: Nan Okun reports funding from Roche Diagnostic, outside the submitted work. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Shelley Dougan, Nan Okun, Kara Bellai-Dussault, Jessica Reszel, Andrea Lanes, Mark Walker and Christine Armour conceptualized the study, which Shelley Dougan, Nan Okun, Kara Bellai-Dussault, Lynn Meng, Tianhua Huang and Christine Armour designed. Shelley Dougan, Kara Bellai-Dussault, Lynn Meng, Tianhua Huang and Christine Armour acquired data, which was analyzed and interpreted by Kara Bellai-Dussault, Lynn Meng and Heather Howley. Shelley Dougan, Nan Okun, Kara Bellai-Dussault, Heather Howley, Jessica Reszel, Andrea Lanes and Christine Armour drafted the manuscript. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work. Shelley Dougan and Nan Okun are co-first authors.
Funding: This study was supported in part by BORN Ontario, which is funded by the Ontario Ministry of Health. Heather Howley is supported by the CHEO Foundation. Mark Walker is a recipient of a Canadian Institutes of Health Research Foundation Grant (FDN No. 148438).
Data sharing: The data set for this study is held securely at the prescribed registry BORN Ontario. Data sharing regulations prevent these data from being made available publicly. Enquiries regarding BORN data may be directed to Science{at}BORNOntario.ca. Please contact Shelley Dougan for more information on accessing BORN data or the study’s programming code.
Disclaimer: Parts of this material are based on data and information compiled and provided by BORN Ontario. The analyses, conclusions, opinions and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred.
- Accepted May 21, 2021.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/