- © 2007 Canadian Medical Association
The case: A 57-year-old man presented to hospital with malaise, abdominal pain and bilateral leg edema. He had immigrated to Canada from St. Vincent, Virgin Islands, 30 years earlier and had last visited the Caribbean 7 years before presentation. The patient's medical history included hypertension, type 2 diabetes and persistent strongyloidiasis.
Initial investigations revealed ascites, abdominal lymphadenopathy, acute renal failure (creatinine 320 μmol/L) and hypercalcemia (calcium 2.8 mmol/L), and he had no eosinophils. The results of a lymph node biopsy suggested a diagnosis of adult T-cell lymphoma. The results of immunophenotyping revealed the presence of CD2 and CD3 (T-cell markers) and CD25 (IL-2 receptor, activated T-cell marker) and the absence of CD5 (B-cell marker) and CD7 (immature T-cell marker). The presence of CD7 is common in cases of acute lymphocytic leukemia; however, this marker is not usually present in cases of adult T-cell lymphoma. The presence of CD25, hypercalcemia and persistent strongyloidiasis and the absence of CD7 were consistent with a diagnosis of T-cell lymphoma caused by human T-cell lymphotropic virus 1 (HTLV-1). The results of serologic investigations were positive for HTLV-1, and the results of a polymerase chain reaction test showed the presence of HTLV-1 proviral DNA.
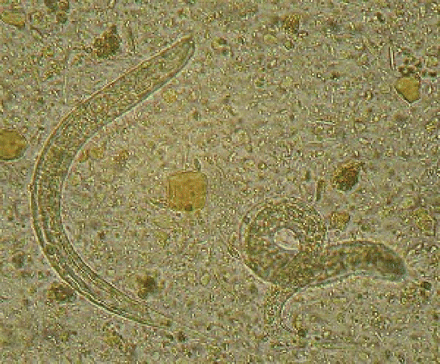
Dialysis was ordered to treat the patient's renal failure and hypercalcemia. After a week of dialysis, his calcium and creatinine levels normalized at 2.1 mmol/L and 86 μmol/L respectively. The patient's lymphoma was treated with cyclophosphamide, hydroxydaunorubicin, vincristine and prednisone (CHOP therapy). In addition, the patient was given a single dose of ivermectin (15 mg) for treatment of strongyloidiasis, which had been diagnosed by the presence of numerous rhabditiform larvae in a duodenal aspirate (Figure 1) 4 weeks before presentation at hospital. The patient's renal function and ascites improved after chemotherapy; however, 8 days after receiving the chemotherapy, fever, diaphoresis and hypotension developed. Blood cultures were positive for Escherichia coli and Citrobacter freundii, and a culture from the patient's central venous catheter was positive for E. coli, C. freundii, Staphylococcus epidermidis, Pseudomonas aeruginosa and α-hemolytic streptococci. Ciprofloxacin, piperacillin–tazobactam and vancomycin therapy were initiated; however, the patient's condition further deteriorated and upper and lower gastrointestinal hemorrhage and cytomegalovirus colitis developed. The patient received blood products, ganciclovir therapy, endoscopic cautery of varices and injection of anhydrous alcohol as a sclerosing agent. Supportive treatments were continued, and the patient was given a second course of ivermectin (15 mg/d for 2 days). Six cycles of chemotherapy were completed; however, a follow-up CT scan of the abdomen showed progressive lymphoma. The patient was given a trial of zidovudine and α-interferon for HTLV-1–associated lymphoma; however, his condition continued to deteriorate, and he died about 6 months after his initial presentation.
Figure 1: Strongyloides stercoralis rhabditiform larvae in the patient's duodenal aspirate.
This case illustrates the potentially deadly interaction between a retrovirus (HTLV-1) that is found in many parts of the world, including Canada, and a parasite (Strongyloides stercoralis) that is ubiquitous in tropical and subtropical regions.
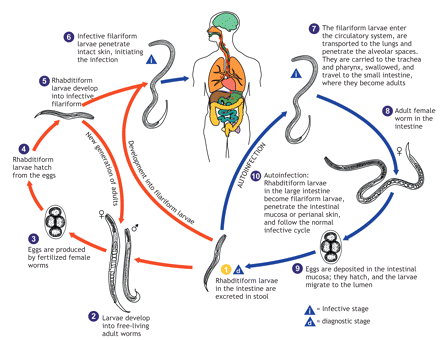
S. stercoralis is a parasitic nematode endemic to tropical and subtropical regions.1 Its life cycle is shown in Figure 2.2 S. stercoralis eggs hatch in the small intestinal mucosa and become rhabdoid larvae that are excreted. The larvae either become free-living worms that produce eggs or become filariform larvae. Filariform larvae from contaminated soil penetrate human skin and are transported to the lungs where they are carried to the pharynx and are swallowed. Once in the intestine, the female adult worms lay their eggs.1 Autoinfection occurs when rhabditiform larvae in the intestine differentiate into filariform larvae, and it may persist for years and even decades after the initial infection.1,3 Autoinfection can lead to hyperinfection syndrome in people who are immunocompromised.1 In hyperinfection, parasites migrate through the gastrointestinal tract and travel throughout the body tissues, potentially causing gram-negative or polymicrobial bacteremia.1,3
Figure 2: Life cycle of Strongyloides stercoralis.2 Photo by: US Centers for Disease Control and Prevention, Alexander J. da Silva, PhD, Melanie Moser.
Symptoms of S. stercoralis infection vary from none to life-threatening sepsis (Box 1).3 Although autoinfection is universal in human hosts, severe hyperinfection syndrome is usually limited to patients with aberrant immune function (Box 2).1,3 Recently, coinfection of S. stercoralis and HTLV-1 has been identified as a major factor related to severe and persistent strongyloidiasis.3
The diagnosis of strongyloidiasis requires either the observation of rhabditiform or filariform S. stercoralis larvae in a stool sample or the appropriate serologic findings.1 A finding of eosinophilia is suggestive of strongyloidiasis, especially if the patient has a history of travel to tropical or subtropical regions. Eosinophilia occurs in > 70% of affected patients.1 An enzyme-linked immunosorbent assay to measure serum antibodies against S. stercoralis is available and has a high sensitivity and specificity, making it a useful diagnostic tool in cases with an unclear diagnosis.1 In Canada, this test is available at the National Reference Centre for Parasitology at McGill University in Montréal.
HTLV-1 is a retrovirus endemic to parts of Africa, the Caribbean, Japan, South America and northern Canada.3,4 Risk factors for acquiring the virus are similar to those for other retroviruses (e.g., HIV). HTLV-1 is transmitted by breast feeding, intercourse and contaminated blood products or instruments.4 It infects T cells and establishes life-long latency.3,4 Infection is typically asymptomatic, but may lead to progressive myelopathy (tropical spastic paraparesis) or T-cell lymphoma that ranges from indolent (smouldering form) to fulminant (acute form).4 Further details about HTLV-1 infection can be found in an excellent review by Verdonck and colleagues.4
The immunology of S. stercoralis and HTLV-1 coinfection is complicated. HTLV-1 causes a generalized activation of the immune system and the disproportionate expression of type 1 cytokines (interferon-γ and tumour necrosis factor-α) by both infected and uninfected T lymphocytes.3,4 In contrast, the control and elimination of helminthic infections requires expression of type 2 cytokines (interleukin-4, interleukin-5, interleukin-10 and interleukin-13), which enhance the normal host response to parasitic infection including eosinophil and IgE production.1,3,4 Because type 1 and type 2 cytokines antagonize each other at the level of cellular expression, coinfected patients have a downregulated response to S. stercoralis.1 Furthermore, eosinophilia and anti-Strongyloides IgE are often absent in coinfected patients owing to interferon-γ–induced suppression of eosinophil maturation.1,3
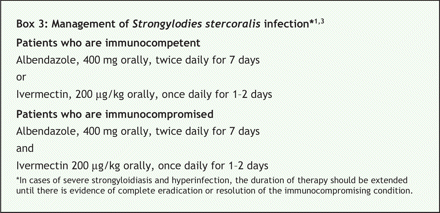
Management of coinfected cases is difficult, and hyperinfection is associated with a high mortality rate.1,3 The antihelminthic drugs albendazole and ivermectin are the mainstay of therapy (Box 3). However, the optimal duration of therapy has not been defined, and complete eradication must be the goal because even a single nematode can initiate autoinfection. Treatment durations as short as 2 days (ivermectin) and 3 days (albendazole) have resulted in clinical success, but microbiologic eradication rates may be as low as 38%.1 Therefore, patients who are immunocompromised and those with HTLV-1 infection or disseminated strongyloidiasis need a longer duration of therapy (often until there is evidence that the parasite is cleared) and combination therapy should be considered.1 Complications of S. stercoralis hyperinfection, including septic shock, intestinal obstruction and pneumonia, are treated conventionally. Management of HTLV-1 infection depends on clinical presentation and ranges from observation to aggressive chemotherapy.4 Antiretroviral drugs (i.e., zidovudine) and interferon-α have been used as adjunctive therapy in refractory cases.4
The most important aspect of the management of S. stercoralis and HTLV-1 coinfection is the timely diagnosis and treatment of S. stercoralis. Those at risk for HTLV-1 infection, such as people from HTLV-1–endemic areas and those with compatible exposure (e.g., intravenous drug use, recipient of blood products, HTLV-1 infected partner or family member) should be tested for HTLV-1 and, if positive, screened for S. stercoralis.1,3 People with persistent S. stercoralis infections should be evaluated for a predisposing condition such as HTLV-1 infection, and people from HTLV-1–endemic areas should be tested for S. stercoralis before the initiation of immunosuppressive therapy.1,3
Clinicians must be aware of this treatable, yet potentially lethal, condition because of increasing rates of immigration and travel and the recent recognition of northern Canada as an HTLV-1–endemic area. Furthermore, clinicians must be aware of other unexpected parasitic infectious diseases that may be acquired abroad and that may present years or even decades later and that can lead to potentially serious conditions (including leishmaniasis, toxoplasmosis, filariasis, trypanosomiasis, infection with liver, lung or blood flukes and nonfalciparum malaria).
Footnotes
-
This article has been peer reviewed.
Competing interests: None declared.