Abstract
Background: In Canada, inactivated hepatitis A vaccines are targeted selectively at those at increased risk for infection or its complications. In order to evaluate the need for routine hepatitis A vaccination programs in Vancouver for street youth, injection drug users (IDUs) and men who have sex with men (MSM), we determined the prevalence of antibodies against hepatitis A virus (HAV) and risk factors for HAV in these groups.
Methods: The frequency of past HAV infection was measured in a sample of Vancouver street youth, IDUs and MSM attending outreach and STD clinics and needle exchange facilities by testing their saliva for anti-HAV immunoglobulin G. A self-administered, structured questionnaire was used to gather sociodemographic data. Stepwise logistic regression was used to evaluate the association between presumed risk factors and groups and past HAV infection.
Results: Of 494 study participants, 235 self-reported injection drug use, 51 were self-identified as MSM and 111 met street youth criteria. Positive test results for anti-HAV were found in 6.3% of street youth (95% confidence interval [CI] 2.6%–12.6%), 42.6% (95% CI 36.2%–48.9%) of IDUs and 14.7% (95% CI 10.4%–19.1%) of individuals who denied injection drug use. Among men who denied injection drug use, the prevalence was 26.3% (10/38) for MSM and 12% (21/175) for heterosexuals. Logistic regression showed that past HAV infection was associated with increased age and birth in a country with high rates of hepatitis infection. Injection drug use among young adults (25–34 years old) was a significant risk factor for a positive anti-HAV test (p = 0.009). MSM were also at higher risk for past HAV infection, although this association was nominally significant (p = 0.07).
Interpretation: Low rates of past HAV infection among Vancouver street youth indicate a low rate of virus circulation in this population, which is vulnerable to hepatitis A outbreaks. An increased risk for HAV infection in IDUs and MSM supports the need to develop routine vaccination programs for these groups also.
Hepatitis A virus (HAV) is the most frequent cause of viral hepatitis. Infection rates have improved through better hygiene and public sanitation, but even within low-risk countries such as Canada and the United States the incidence of infection remains heterogeneous across geographic and socioeconomic strata.1,2,3 The availability of effective inactivated vaccines provides a powerful new tool to enhance HAV control. Universal vaccination programs for children and youth are being designed and implemented in the United States in areas of increased hepatitis A incidence.4 In Canada, the vaccine is still targeted selectively at those at increased risk for infection or its complications.
Recurrent hepatitis A outbreaks among men who have sex with men (MSM) and injection drug users (IDUs) have contributed significantly to Vancouver's high hepatitis A incidence rates of 24–27 cases per 100 000 population.3,5 These exceed the provincial average by up to 3 times.
Within the past decade, the health risks of street youth have been studied extensively, yet the prevalence of HAV infection in this population in developed countries has not been investigated. Youth living on the streets may be at increased risk for HAV infection as they are frequently involved in behaviours associated with higher risk, such as injection drug use6,7 or involvement in the sex trade, resulting in multiple sexual partners.8,9 Street youth often have restricted access to adequate sanitary facilities.
This study explored the risk of HAV infection among Vancouver's street youth, IDUs and MSM through testing for the presence of HAV-specific immunoglobulin G in their saliva. In order to assess the need for routine vaccination programs in these communities, we evaluated whether being in each category (MSM, IDUs, street youth) constitutes an independent risk factor for HAV infection. The beginning of study enrolment coincided with the launch of a public health effort in the Greater Vancouver area to provide one dose of hepatitis A vaccine to Vancouver's MSM and IDUs as an outbreak control measure.
Methods
Study recruitment was conducted over a 6-month period from March to August 1998 through 4 locations in Greater Vancouver: 2 “street outreach clinics” operating in the Vancouver downtown area, one of which mainly serves a street youth population, whereas the other clinic, which is located near to the Vancouver needle exchange facility, was frequented predominantly by injection drug users; a needle exchange facility serving injection drug users in Surrey; and an STD clinic located in Vancouver.
All those who attended the outreach clinics and the needle exchange facility were invited to participate in this study, whereas at the STD clinic the invitation was extended only to males. This restriction was dictated by the feasibility of securing a sufficient sample of MSM and heterosexual men who did not use drugs and were of similar social status. Excluded from the study were those individuals who said that they had already been immunized against hepatitis A. At each location, recruitment was conducted until about 120 individuals were enrolled. The rate of refusal to participate in the study was not recorded.
Saliva collection was facilitated by the use of Salivette (Sarstedt, Rommelsdorf, Germany) with a neutral cotton insert. Study participants were asked to place the insert into the mouth, chewing occasionally until the insert was wet. Within 24 hours of collection, the saliva was recovered by centrifugation, divided into aliquots and frozen at –70° C until assayed. The presence of HAV-specific IgG was evaluated using an ultrasensitive capture enzyme immunoassay–based method, which demonstrates 99% sensitivity and specificity when compared with serum-based tests.10
Information about demographics, sexual orientation and practices, previous injection drug use and hours per day spent on the streets was collected using a 1-page, self-administered, structured questionnaire. The questionnaire was based on a similar instrument used successfully to survey Vancouver MSM at the onset of a hepatitis A outbreak. The study design, including the questionnaire, was reviewed and approved by the Clinical Research Ethics Board at the University of British Columbia
Individuals who reported having injected drugs in the past were identified as injection drug users (IDUs), whereas males who reported a homosexual or bisexual orientation were identified as men who have sex with men (MSM). To qualify as street youth, respondents had to be less than 25 years of age and had to have reported spending at least 8 hours daily on the streets.
The association between potential risk factors and groups and past infection with HAV was evaluated using stepdown logistic regression. Goodness-of-fit statistics were examined to determine the appropriateness of the final model.
Besides the 3 groups (MSM, IDUs and street youth) who were originally thought to be at increased risk for HAV infection, the initial model also included sex, age group and birth in a country where there was an increased risk of HAV infection, the last 2 being well-recognized factors associated with past HAV infection. The 2-way interactions between age and hypothesized risk groups were examined, as were the 2- and 3-way interactions among the risk groups themselves. The site of recruitment was not considered to be a separate risk factor, because it was self-evident that there was a substantial overlap of the risk groups under investigation due to the specialized nature of these clinics.
Results
A total of 494 individuals were recruited into the study (Table 1). The majority (77.5%) of the 111 study participants who met street youth criteria were enrolled through a street youth–oriented, downtown outreach clinic, whereas most of the 235 subjects who reported past injection drug use were recruited through the needle exchange facility (41.7%) and through the second downtown outreach clinic (42.1%). An MSM orientation was reported by 51 males, most of whom were recruited at the STD clinic (54.9%) and at the youth-oriented outreach clinic (25.5%). Most (71%) of the 157 participants who did not fall into any of the 3 risk groups were recruited at the STD clinic.
Table 1.
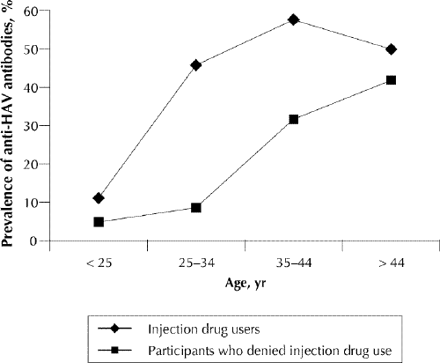
The presence of HAV-specific IgG was detected in saliva specimens from 138 individuals, resulting in an overall prevalence rate of 27.9% (Table 1). Evidence of past HAV infection was detected in 7 of 111 participants who met the street youth criteria, indicating a prevalence of 6.3% (95% confidence interval [CI] 2.6–12.6). Street youth who were IDUs had higher rates of past infection with hepatitis A (4/42, 9.5%, 95% CI 2.7%–22.6%) compared with street youth who denied a history of injection drug use (3/69, 4.3%, 95% CI 0.9%–12.2%), although these differences were not statistically significant. The presence of anti-HAV was almost 3 times more common among all IDUs (100/235, 42.6%, 95% CI 36.2%–48.9%) than among subjects who denied injection drug use (38/258, 14.7%, 95% CI 10.4%–19.1%). The differences in rates of previous HAV infection among IDUs and non-IDUs were most pronounced in those aged 25–34 and 35–44 years and not present among those aged 45 years and above, as illustrated in Fig. 1. Anti-HAV prevalence among MSM (13/51, 25.5%) was almost identical to that of heterosexual men (83/315, 26.3%). After the exclusion of IDUs, however, it was more than twice as high among MSM (10/38, 26.3%) than among heterosexuals (21/175, 12%).
Fig. 1: Prevalence of antibodies against hepatitis A virus (HAV) according to age and self-reported injection drug use.
Logistic regression identified 2 factors to be significantly associated with increased anti-HAV prevalence (Table 2): most obviously with greater age but also with birth in a country where there was increased risk for HAV infection. IDUs and MSM were also at higher risk for past HAV infection, although these associations were nominally significant.
Table 2.
The regression analysis also identified 2 interactions that were significantly associated with increased anti-HAV prevalence. First, an interaction between IDUs and age (p = 0.05) indicates that injection drug use especially among young adults (25–34 years old) is a significant risk factor for a positive anti-HAV test (p = 0.009). Second, a significant interaction between IDUs and MSM (p = 0.03) indicates that among MSM injection drug use is not associated with the increased presence of anti-HAV. Among those who are not MSM, however, the anti-HAV rate was 3.9 times higher in IDUs than in those who denied injection drug use (95/218, 43.6%) versus (24/213, 11.3%).
Interpretation
Our findings stress once again that injection drug use is not only associated with an unfavourable course of HAV infection due to the likelihood of underlying chronic liver diseases,11,12 but also with an increased risk for HAV infection itself, particularly among young adults. The stable, as opposed to increasing, prevalence of anti-HAV in the older IDUs could reflect a past pattern of risk reduction behaviour and the cyclic nature of HAV transmission. The development and implementation of a routine vaccination program for IDUs appears to be the only rational approach to breaking the cycle of hepatitis A outbreaks and, in the long run, saving costs and health care resources. Similarly, in the case of MSM, our limited data tend to support calls for a routine 2-dose vaccination program to provide long-term protection, as opposed to sporadic 1-dose vaccine interventions as an outbreak control measure requiring sudden bursts of public health activity.
The high, age-independent HAV infection rate observed among IDUs in Vancouver is in accordance with previous reports associating injection drug use with increased risk for this infection.13,14,15 The difference in anti-HAV rates between young adult IDUs and those who denied prior injection drug use should be interpreted with some caution. The majority of participants who denied injection drug use were enrolled into the study through clinics other than the needle exchange program, which also included an STD clinic located in a more affluent Vancouver neighbourhood. It is possible that the observed low rate in this group reflects not only lack of involvement in injection drug use but also different social background. Socioeconomic status is a significant factor associated with the prevalence of HAV infection,16 and it could not be controlled for in our study.
Discrepancies in social background, however, are unlikely to play any significant role in the difference in anti-HAV prevalence observed between MSM and heterosexual men, because the proportion of individuals enrolled through the STD clinic into each group was almost identical. The similar rates of anti-HAV prevalence among MSM and heterosexual men are probably the result of there being a higher proportion of IDUs among the heterosexual men (44%) than among MSM (25%) and the lack of any additional effect of injection drug use on anti-HAV rates in MSM. The absence of additional risk may be because the harm reduction message that has been delivered to MSM has spilled over into their injection drug–using habits. Although the difference in anti-HAV rate between MSM and heterosexual men was more than 2-fold when individuals who denied injection drug use were considered, being MSM on its own did not emerge as a significant risk factor in logistic regression. This result may have been a consequence of the relatively small number of MSM studied (51); they constituted only about 10% of study participants, and of these only 33 denied injection drug use and were not street youth.
Evidence of past HAV infection was present in only 6.3% of Vancouver street youth. This closely resembles the anti-HAV prevalence of 7.1% (95% CI 4.1%–11.3%) detected 2 years earlier among grade six students in Vancouver.17 More than 25% of the sixth graders, however, were born outside Canada, and this subgroup contained the majority of anti-HAV-positive children, whereas the sample of street youth included only 5 individuals born elsewhere. Although the anti-HAV positivity rate among Canadian-born street youth (6.6%) was twice as high as the rate observed among Canadian-born grade six students (3%), it still should be considered to be relatively low. Such a low rate means that almost all of Vancouver street youth are likely to be susceptible to HAV. When this vulnerability is considered in addition to the substandard living conditions of a high proportion of this population and their exposure to other risk factors associated with HAV infection, such as injection drug use (often resulting in needle sharing6) and involvement in the sex trade (“survival sex,” multiple sexual partners), it would seem that an outbreak of hepatitis A is just waiting to happen. The design and implementation of a routine hepatitis A vaccination program for street youth is challenging, because it is difficult to reach all of this elusive and dynamic population, but street youth do tend to concentrate in relatively small areas in certain parts of the city. However, they represent a heterogeneous group whose time spent on the streets may vary considerably.
Reports from countries such as Brazil where there is an increased risk of contracting HAV infection indicate a very high prevalence of 80%–92% in street youth.18 The prevalence of HAV infection in street youth in other Canadian urban populations or in other low-risk countries might be very different from Vancouver's, reflecting local outbreak histories. Such outbreaks involving street youth, however, might be difficult to recognize and attribute to this population, because it is elusive, it overlaps considerably with IDUs and it is often characterized by limited access to health care services. The availability of a vaccine, which is easy to use and, thus, a very effective preventive tool, calls for investigation of HAV prevalence in street youth populations particularly in low-risk countries that have not adopted universal hepatitis A vaccination programs for children and youth.
Acknowledgments
We are indebted to the nursing staff of Vancouver Outreach and STD clinics and the Surrey Needle Exchange for their work in study enrolment, supervision of specimen collection and data gathering. We also thank Dr. Robert Strang, Dr. Danuta Skowronski and Mr. Jim Bennett for their continued support throughout the study in allowing access to clinics.
This work was supported by a British Columbia Health Research Foundation grant.
Footnotes
-
This article has been peer reviewed.
Contributors: Drs. Ochnio, Patrick and Dobson contributed to the design and execution of this study. Ms. Ho was responsbility for the accuracy of laboratory results and for data analysis. Mr. Talling designed the logistic regression model used for evaluation of risk factors. All the authors took part in the drafting and revising of the article.
Competing interests: None declared.