Most patients do not look forward to injections, particularly when required repeatedly for chronic diseases. For years, alternatives to injections have been sought for therapy with large molecules such as peptides and proteins. Oral, nasal and transdermal routes of delivery have been found to be generally ineffective and inefficient. Most macromolecules are too large to pass naturally (i.e., without penetration enhancers) in therapeutic doses through skin, nasal membranes or the gastrointestinal tract, where they may be destroyed by gastric juices or pancreatic enzymes.
Lung epithelial permeability: the lung-blood barrier
Pulmonary delivery offers a noninvasive option for systemic therapy with large molecules such as insulin, growth hormone, interferon-β and calcitonin. Numerous studies have shown that the lungs are efficiently permeable for delivering these and other large molecules into the circulation.[1–4] Medication consisting of a fine particle aerosol is carried to the alveolar epithelium, whose surface area is about 100 m2 in adults (the size of a singles tennis court) during slow deep inhalation. The 500 million alveoli are enveloped by an equally large capillary network through which many proteins and peptides and, of course, small molecules can be readily absorbed into the bloodstream.
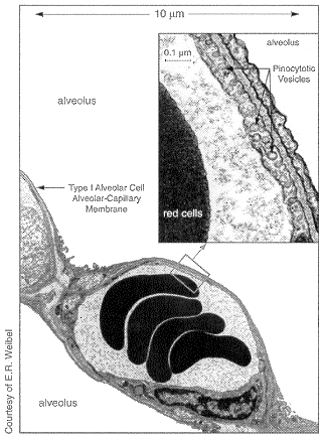
Studies suggest that peptides and proteins are absorbed into the bloodstream via transcytosis,5 a natural process that occurs deep in the lungs (Fig. 1). Large drug particles are transported fairly rapidly across the impermeable cell membrane of the alveolar epithelium by a "bucket brigade" of tiny membrane bubbles, or transcytotic vesicles, formed by invagination of the cell membrane.
Fig. 1: Potential site of drug delivery for systemic disease. Through transcytosis, large drug particles would be transported across cell membranes of alveolar epithelium by billions of vesicles.
Alveolar deposition efficiency and transalveolar absorption of proteins and peptides into the lung capillaries depend on several factors, including aerosol particle size, molecular weight of the medication, solubility, particle charge, lipophilicity and epithelial membrane permeability. With epithelial inflammation, permeability is much greater, as seen in people who smoke and patients with lung fibrosis. Physiologic factors such as regional blood flow and respiratory variables such as inspiratory capacity or tidal volume breaths, inspiratory and expiratory flow rate and duration of breath-hold at total lung capacity are also important. These parameters determine the efficiency of aerosol deposition in the alveolar epithelium and the transfer of the drug molecules into the regional capillaries.
Aerosol technology for improved delivery to the deep lung
One of the most important factors determining efficiency of drug delivery to the alveolar epithelium is particle size. Ideally, the drug particles should be 1-3 μm in aerodynamic diameter, and, because the inspiratory flow rate is also critical for targeting the alveoli, they should be delivered by slow, deep inhalation. In adults the probability of particles 5-10 μm in aerodynamic diameter reaching the lower respiratory tract is less than 10% at an inspiratory flow rate of 30 L/min, with very few reaching the peripheral regions of the lungs. Smaller particles (less than 2 μm) have about an 80% chance of reaching the lower respiratory tract, with 50%-60% being deposited in nonciliated airways and alveoli.[1–4]
Even relatively insoluble dry-powder aerosols quickly dissolve in the fluid lining the epithelium, mainly because of the enormous surface area. This is noteworthy because dry powders have several important advantages over liquid systems: they enable stable formulations at room temperature, deliver a high dose per puff and, during storage, resist microbial growth.
The potential of aerosol macromolecule delivery was recently emphasized when results were reported in 1998 from phase II trials of inhaled insulin in the United States.[6–8] These 12-week clinical trials compared short-acting injected insulin with short-acting inhaled insulin taken before meals by both patients with type 1 and type 2 diabetes mellitus. The level of glycemic control did not differ statistically between the 2 formulations. Phase III trials involving people with type 1 and type 2 diabetes at 117 sites are currently under way.
Safety issues
The lung is by necessity a remarkably robust organ accustomed to the daily inhalation of large numbers of particles. In 24 hours an average adult inhales about 20 000 L of air containing a variety of biological and nonbiological particulate pollutants. In 1992-93 the American Conference of Governmental Industrial Hygienists determined the threshold limit values for inhalation, including the limit of insoluble "nuisance dust" that can be inhaled, over the long term without measurable injury.9 They agreed that workers can inhale 30 mg of nuisance dust in an 8-hour work day over many years without negative effect. By comparison, inhaled macromolecules such as insulin, which are fairly rapidly absorbed from the epithelial surface, will deposit no more than about 10 mg on average (range 1-20 mg). For example, each average dose of 1-2 mg (3-6 units) of inhaled insulin provides 300-500 × 106 particles, or about 1 particle per alveolus. In the 3-month phase II trials, the inhaled insulin was very well tolerated and showed no adverse effects on standard pulmonary function tests.[6–8]
In addition, self-proteins such as those of inhaled insulin do not tend to cause an immune response. Aerosol vaccines, where an immune response is the intended effect, are made with foreign proteins.
A new treatment paradigm
For indications such as multiple sclerosis, osteoporosis, α1-antitrypsin deficiency emphysema, infertility and growth defects - where drugs today must be injected - the hope is that many patients will eventually be able to inhale their medications. In the United States alone, there are more than 30 approved injectable macromolecular drugs and more than 100 others currently in the pipeline. Many of these will probably be suitable for aerosol administration.
The easy-to-use pulmonary delivery system should encourage patient compliance by providing a noninvasive method of administering drugs, particularly for patients with chronic diseases. Diabetic patients will be more inclined to take their insulin, which would thus improve glycemic control and thereby decrease the incidence of diabetic retinopathy and nephropathy, and perhaps other complications of poorly controlled diabetes as well. The huge and very thin (0.1-0.2 mm) epithelial surface provided by the alveoli is a potential route for easy, convenient and painless drug administration, which should result in aerosol solutions to many medical problems - a new treatment paradigm for the next millennium.
Competing interests: Dr. Newhouse is employed by and has stock options in Inhale Therapeutic Systems, San Carlos, Calif., a manufacturer of aerosol drugs and devices. He has received consultancy fees, contract funds, speaker fees and travel assistance from various pharmaceutical companies.
Footnotes
-
This article has been peer reviewed.
Reprint requests to: Joyce Strand, Inhale Therapeutic Systems, 150 Industrial Rd., San Carlos CA 94070-6256; fax 650 631-3150