Bayer's abrupt worldwide withdrawal of Baycol (cerivastatin) in August 2001 came as a surprise given that rhabdomyolysis is a side effect common to all statins.1,2 We questioned whether Bayer's introduction of newer strengths of cerivastatin (Baycol-4, Baycol-8) may have inadvertently led to excessive dosing of cerivastatin and an increased occurrence of rhabdomyolysis.
We obtained data on all 47 cases of rhabdomyolysis associated with cerivastatin from the Marketed Health Products Directorate of Health Canada, including the number of cases occurring for each daily dose of the medication. Given that the number of patients taking any given strength of the drug is not known, we calculated the number of days each product had been on the market (from its date of launch to Aug. 24, 2001) as a surrogate measure of exposure to the product. We then calculated the probability of rhabdomyolysis per day that each product was on the market (Table 1).
Table 1.
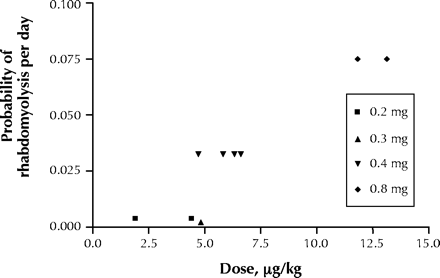
For the 11 cases where the patient's weight was provided, we calculated the daily dose (in μg/kg body weight). Graphic presentation of the probability of rhabdomyolysis (per day) in relation to cerivastatin dose in μg/kg body weight (Fig. 1) demonstrates that patients taking a higher daily dose (typically from the use of higher-strength products) were much more likely to develop rhabdomyolysis. The actual dose in μg/kg ranged from 1.9 μg to 13.1 μg (a 7-fold difference), while the available strengths ranged from 0.2 mg to 0.8 mg (only a 4-fold difference).
Fig. 1: Probability of rhabdomyolysis (per day that each product was on the market) in relation to cerivastatin dose in mg/kg body weight.
It is likely that these high doses are sufficient to cause rhabdomyolysis even in the absence of concomitant fibrate therapy. On this point, there were 28 cases of rhabdomyolysis associated with the 0.2 mg, 0.3 mg, and 0.4 mg daily doses; 15 (54%) of these patients also received a concomitant fibrate. However, of the 18 patients taking the 0.8 mg daily dose, only 3 (17%) received a concomitant fibrate.
Our data have some obvious limitations. The voluntary nature of adverse events reporting likely understates the magnitude of the problem. In addition, only 11 out of 47 reports recorded patient weight, limiting the analysis of the rate of rhabdomyolysis and dose. Nevertheless, the cerivastatin story suggests that dosing information from the manufacturers of all drugs, regardless of class, should be in terms of mg/kg of body weight. This would provide a better guide for the clinician in choosing the most appropriate medication strength for a given patient. The prescription of standard medication strengths, while ignoring a patient's body mass, may lead to the use of inappropriately high medication doses, preventable medication-related adverse events and the withdrawal from the market of otherwise useful medication.
Dipen Kalaria Director, Pharmacy Services Willem Wassenaar Medical Director Pharmacy.ca Toronto, Ont.