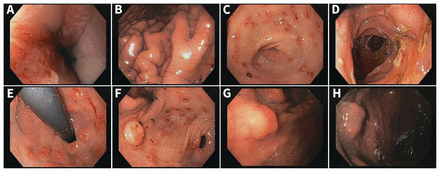
A 25-year-old man previously diagnosed with irritable bowel syndrome (2–3 loose stools per day) presented for esophagogastroduodenoscopy because of increasing epigastric discomfort. Numerous peptic complications were found during the procedure (Figure 1A–1D). We scrutinized the bulb meticulously (Figure 1E) but did not find a neuroendocrine tumour (NET) lesion there; however, we did find an eroded lesion (Figure 1F) at the angularis. Biopsy of the lesion showed a well-differentiated G1 NET with a proliferative index of less than 2%. Test results for Helicobacter pylori were negative. The patient’s gastric pH was 1. A gastrin analysis before initiation of proton pump inhibitors (PPIs) showed an elevated fasting level of 573 (assay-specific normal range < 115) ng/L. Secretin stimulation testing (2 IU/kg) showed a delta of 1685 (normal range < 120) ng/L,1 confirmative of Zollinger–Ellison syndrome.
Upper esophagogastroduodenoscopy views in a 25-year-old man with Zollinger–Ellison syndrome caused by a gastric gastrinoma. (A) Distal esophagus with severe stricturing reflux esophagitis. (B) Gastric body showing hyperplastic gastropathy. (C) Erosive lesions in the gastric antrum. (D) Superficial and partly necrotic ulcers in the postbulbar duodenum, predominantly on the folds. (E) Retroflexed view of the postpyloric duodenal bulb with heterotopic gastric mucosa with inflammatory changes. (F) Superficially eroded subepithelial lesion (about 7 mm in size) at the angularis, confirmed as a well-differentiated neuroendocrine tumour. (G) Gastric gastrinoma on white light and (H) narrow-band imaging after reversal of peptic changes by treatment with proton pump inhibitors.
Upon treatment with pantoprazole (40 mg twice a day), the patient’s stool frequency normalized, and the peptic lesions healed completely within 4 weeks, with the primary lesion more clearly demarcated (Figure 1G, 1H).2 After further work-up including endoscopic ultrasonography, DOTATATE–positron emission tomography–computed tomography and negative results for biochemical and genetic testing for multiple endocrine neoplasia type 1, the patient underwent distal gastrectomy with radical D2 dissection for marked lymph node involvement.
Zollinger–Ellison syndrome is an uncommon diagnosis (estimated incidence of 1 in 1 million). Peptic complications may be underdiagnosed, because PPIs can obscure the clinical presentation. 3 Although extragastric gastrinomas may give rise to gastric (type 2) NETs secondary to chronic hypergastrinemia, postoperative biochemical remission of gastrin excess in our patient again confirmed a primary location outside the so-called gastrinoma triangle.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
The authors have obtained patient consent.