Abstract
Introduction: Human papillomavirus (HPV) is one of the world’s most common sexually transmitted infections, and has been associated with a number of cervical and non-cervical diseases, including cancer. HPV vaccines have been licensed for use in females for some time, but the quadrivalent vaccine has only recently become licensed for use in males. Many countries have adopted a vaccination programme for adolescent females based on results of cost-effectiveness analyses. However, given the new indications for use of the vaccine in males, decision makers require information on the cost effectiveness of vaccinating males in order to make policy decisions on whether or not to fund such programmes.
Objective: Our objective was to conduct a qualitative systematic review to update a previously conducted review of HPV vaccine studies.
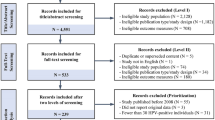
Methods: Articles were obtained from an extensive literature search to determine the cost effectiveness of implementing an HPV vaccination programme with routine cervical cancer screening. A total of 29 studies were included in this review. Seventeen of the included articles looked only at cervical disease outcomes, and 12 studies also included non-cervical disease outcomes. Four studies explored the economic impact of vaccinating both boys and girls. One study focused on a population of men who have sex with men (MSM).
Results: While different model structures, input parameters and baseline assumptions were used, the consistent message in studies that focused on female-only vaccination programmes was that routine vaccination of females is cost effective compared with cervical cancer screening alone.
Discussion: Based on the currently available literature, it appears that the addition of boys to a vaccination programme generally exceeds traditional cost-effectiveness thresholds. The MSM population represents a potential additional target for routine HPV vaccination; however, more cost-effectiveness studies are required before making such a policy change.



Similar content being viewed by others
References
Smith JS, Lindsay L, Hoots B, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer 2007; 121: 621–32
Parkin DM, Bray F. Chapter 2: the burden of HPV-related cancers. Vaccine 2006; 24 Suppl. 3: S3/11–25
Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer 2006; 118: 3030–44
Goodman A. Primary vaginal cancer. Surg Oncol Clin N Am 1998; 7: 347–61
Jones RW, Rowan DM, Stewart AW. Vulvar intraepithelial neoplasia: aspects of the natural history and outcome in 405 women. Obstet Gynecol 2005; 106: 1319–26
Dianzani C, Calvieri S, Pierangeli A, et al. Identification of human papilloma viruses in male dysplastic genital lesions. New Microbiol 2004; 27: 65–9
McKaig RG, Baric RS, Olshan AF. Human papillomavirus and head and neck cancer: epidemiology and molecular biology. Head Neck 1998; 20: 250–65
Lacey CJ, Lowndes CM, Shah KV. Chapter 4: burden and management of non-cancerous HPV-related conditions. HPV-6/11 disease. Vaccine 2006; 24 Suppl. 3: S3/35–41
Wiley D, Masongsong E. Human papillomavirus: the burden of infection. Obstet Gynecol Surv 2006; 61: S3–14
Derkay CS, Darrow DH. Recurrent respiratory papillomatosis. Ann Otol Rhinol Laryngol 2006; 115: 1–11
GlaxoSmithKline. Cervarix™: human papillomavirus vaccine types 16 and 18 (recombinant, AS04 adjuvanted) [product monograph; online]. Available from URL: http://www.gsk.ca/english/docs-pdf/product-monographs/Cervarix.pdf [Accessed 2010 Nov 9]
Merck Frosst. Gardasil®: quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine [product monograph; online]. Available from: http://www.merck.ca/mfcl/en/corporate/products/gardasil.html [Accessed 2010 Nov 9]
Harper DM, Franco EL, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 2004; 364: 1757–65
Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367: 1247–55
Mao C, Koutsky LA, Ault KA, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol 2006; 107: 18–27
Villa LL, Ault KA, Giuliano AR, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus types 6, 11, 16, and 18. Vaccine 2006; 24: 5571–83
Villa LL, Costa RL, Petta CA, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol 2005; 6: 271–8
Villa LL, Costa RL, Petta CA, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer 2006; 95: 1459–66
FUTURE I/II Study Group, Dillner J, Kjaer SK, et al. Four year efficacy of prophylactic human papillomavirus quadrivalent vaccine against low grade cervical, vulvar, and vaginal intraepithelial neoplasia and anogenital warts: randomised controlled trial. BMJ 2010; 341: c3493
Koutsky LA, Ault KA, Wheeler CM, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med 2002; 347: 1645–51
FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356: 1915–27
Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007; 356: 1928–43
Giuliano AR, Palefsky J. The efficacy of quadrivalent HPV (types 6/11/16/18) vaccine in reducing the incidence of HPV-related genital disease in young men [online]. Available from URL: http://www.eurogin.com/2008/EUROGIN2008_LastMinuteAbstracts.pdf [Accessed 2010 Nov 9]
Palefsky JM, Giuliano AR. Efficacy of the quadrivalent HPV vaccine against HPV 6/11/16/18-related genital infection in young men [online]. Available from URL: http://www.eurogin.com/2008/EUROGIN2008_LastMinuteAbstracts.pdf [Accessed 2010 Nov 9]
Markowitz LE, Dunne EF, Saraiya M, et al. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56: 1–24
Insinga RP, Dasbach EJ, Elbasha EH. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus-related disease in the US: analytic framework and review of the literature. Pharmacoeconomics 2005; 23(11): 1107–22
Fleurence RL, Dixon JM, Milanova TF, et al. Review of the economic and quality-of-life burden of cervical human papillomavirus disease. Am J Obstet Gynecol 2007; 196: 206–12
Hu D, Goldie S. The economic burden of noncervical human papillomavirus disease in the united states. Am J Obstet Gynecol 2008; 198: 500.e1-7
Sanders GD, Taira AV. Cost-effectiveness of a potential vaccine for human papillomavirus. Emerg Infect Dis 2003; 9: 37–48
Kulasingam SL, Myers ER. Potential health and economic impact of adding a human papillomavirus vaccine to screening programs. JAMA 2003; 290: 781–9
Goldie SJ, Kohli M, Grima D, et al. Projected clinical benefits and cost-effectiveness of a human papillomavirus 16/18 vaccine. J Natl Cancer Inst 2004; 96: 604–15
Brisson M, Van de Velde N, De Wals P, et al. The potential cost-effectiveness of prophylactic human papillomavirus vaccines in Canada. Vaccine 2007; 25: 5399–408
Kulasingam S, Connelly L, Conway E, et al. A cost-effectiveness analysis of adding a human papillomavirus vaccine to the Australian national cervical cancer screening program. Sex Health 2007; 4: 165–75
Giuliano AR. Human papillomavirus vaccination in males. Gynecol Oncol 2007; 107: S24–6
Garnett GP. Role of herd immunity in determining the effect of vaccines against sexually transmitted disease. J Infect Dis 2005; 191 Suppl. 1: S97–106
Marra F, Cloutier K, Oteng B, et al. Effectiveness and cost effectiveness of human papillomavirus vaccine: a systematic review. Pharmacoeconomics 2009; 27(2): 127–47
Drummond MF, Richardson WS, O’Brien BJ, et al. Users’ guides to the medical literature: XIII. How to use an article on economic analysis of clinical practice. A: are the results of the study valid? Evidence-based medicine working group. JAMA 1997; 277: 1552–7
Ginsberg GM, Fisher M, Ben-Shahar I, et al. Cost-utility analysis of vaccination against HPV in Israel. Vaccine 2007; 25: 6677–91
Dasbach EJ, Insinga RP, Elbasha EH. The epidemiological and economic impact of a quadrivalent human papillomavirus vaccine (6/11/16/18) in the UK. BJOG 2008; 115: 947–56
Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med 2008; 359: 821–32
Kim JJ, Kobus KE, Diaz M, et al. Exploring the cost-effectiveness of HPV vaccination in Vietnam: insights for evidence-based cervical cancer prevention policy. Vaccine 2008; 26: 4015–24
Jit M, Choi YH, Edmunds WJ. Economic evaluation of human papillomavirus vaccination in the United Kingdom. BMJ 2008; 337: a769
Rogoza RM, Ferko N, Bentley J, et al. Optimization of primary and secondary cervical cancer prevention strategies in an era of cervical cancer vaccination: a multi-regional health economic analysis. Vaccine 2008; 26 Suppl. 5: F46–58
Diaz M, Kim JJ, Albero G, et al. Health and economic impact of HPV 16 and 18 vaccination and cervical cancer screening in India. Br J Cancer 2008; 99: 230–8
Usher C, Tilson L, Olsen J, et al. Cost-effectiveness of human papillomavirus vaccine in reducing the risk of cervical cancer in Ireland due to HPV types 16 and 18 using a transmission dynamic model. Vaccine 2008; 26: 5654–61
Zechmeister I, Blasio BF, Garnett G, et al. Cost-effectiveness analysis of human papillomavirus-vaccination programs to prevent cervical cancer in Austria. Vaccine 2009; 27: 5133–41
Coupé VM, van Ginkel J, de Melker HE, et al. HPV16/18 vaccination to prevent cervical cancer in the Netherlands: model-based cost-effectiveness. Int J Cancer 2009; 124: 970–8
Annemans L, Remy V, Oyee J, et al. Cost-effectiveness evaluation of a quadrivalent human papillomavirus vaccine in Belgium. Pharmacoeconomics 2009; 27: 231–45
Kim JJ, Goldie SJ. Cost effectiveness analysis of including boys in a human papillomavirus vaccination programme in the United States. BMJ 2009; 339: b3884
Kim JJ, Ortendahl J, Goldie SJ. Cost-effectiveness of human papillomavirus vaccination and cervical cancer screening in women older than 30 years in the United States. Ann Intern Med 2009; 151: 538–45
Thiry N, De Laet C, Hulstaert F, et al. Cost-effectiveness of human papillomavirus vaccination in Belgium: do not forget about cervical cancer screening. Int J Technol Assess Health Care 2009; 25: 161–70
Rogoza RM, Westra TA, Ferko N, et al. Cost-effectiveness of prophylactic vaccination against human papillomavirus 16/18 for the prevention of cervical cancer: adaptation of an existing cohort model to the situation in the Netherlands. Vaccine 2009; 27: 4776–83
de Kok IM, van Ballegooijen M, Habbema JD. Cost-effectiveness analysis of human papillomavirus vaccination in the Netherlands. J Natl Cancer Inst 2009; 101: 1083–92
Colantonio L, Gomez JA, Demarteau N, et al. Cost-effectiveness analysis of a cervical cancer vaccine in five Latin American countries. Vaccine 2009; 27: 5519–29
Sinanovic E, Moodley J, Barone MA, et al. The potential cost-effectiveness of adding a human papillomavirus vaccine to the cervical cancer screening programme in South Africa. Vaccine 2009; 27: 6196–202
Reynales-Shigematsu LM, Rodrigues ER, Lazcano-Ponce E. Cost-effectiveness analysis of a quadrivalent human papilloma virus vaccine in Mexico. Arch Med Res 2009; 40: 503–13
Anonychuk AM, Bauch CT, Merid MF, et al. A cost-utility analysis of cervical cancer vaccination in preadolescent Canadian females. BMC Public Health 2009; 9: 401
Mennini FS, Giorgi Rossi P, Palazzo F, et al. Health and economic impact associated with a quadrivalent HPV vaccine in italy. Gynecol Oncol 2009; 112: 370–6
Dee A, Howell F. A cost-utility analysis of adding a bivalent or quadrivalent HPV vaccine to the Irish cervical screening programme. Eur J Public Health 2010; 20: 213–9
Dasbach EJ, Nagy L, Brandtmuller A, et al. The cost effectiveness of a quadrivalent human papillomavirus vaccine (6/11/16/18) in Hungary. J Med Econ 2010; 13: 110–8
Elbasha EH, Dasbach EJ. Impact of vaccinating boys and men against HPV in the United States. Vaccine 2010; 28: 6858–67
Diaz M, de Sanjose S, Ortendahl J, et al. Cost-effectiveness of human papillomavirus vaccination and screening in Spain. Eur J Cancer 2010; 46: 2973–85
Torvinen S, Nieminen P, Lehtinen M, et al. Cost effectiveness of prophylactic HPV 16/18 vaccination in Finland: results from a modelling exercise. J Med Econ 2010; 13: 284–94
Liu PH, Hu FC, Lee PI, et al. Cost-effectiveness of human papillomavirus vaccination for prevention of cervical cancer in Taiwan. BMC Health Serv Res 2010; 10: 11
Olsen J, Jepsen MR. Human papillomavirus transmission and cost-effectiveness of introducing quadrivalent HPV vaccination in Denmark. Int J Technol Assess Health Care 2010; 26: 183–91
Kim JJ. Targeted human papillomavirus vaccination of men who have sex with men in the USA: a cost-effectiveness modelling analysis. Lancet Infect Dis 2010; 10(12): 845–52
Debicki D, Ferko N, Demarteau N, et al. Comparison of detailed and succinct cohort modelling approaches in a multi-regional evaluation of cervical cancer vaccination. Vaccine 2008; 26 Suppl. 5: F16–28
Kohli M, Ferko N, Martin A, et al. Estimating the long-term impact of a prophylactic human papillomavirus 16/18 vaccine on the burden of cervical cancer in the UK. Br J Cancer 2007; 96: 143–50
Goldie SJ, Kim JJ, Kobus K, et al. Cost-effectiveness of HPV 16, 18 vaccination in Brazil. Vaccine 2007; 25: 6257–70
Kim JJ, Kuntz KM, Stout NK, et al. Multiparameter calibration of a natural history model of cervical cancer. Am J Epidemiol 2007; 166: 137–50
National Board of Health, Danish Centre for Health Technology Assessment. Reduction in the risk of cervical cancer by vaccination against human papillomavirus (HPV): a health technology assessment. Copenhagen: National Board of Health, Danish Centre for Health Technology Assessment, 2007 [online]. Available from URL: http://www.sst.dk/publ/Publ2007/MTV/HPV/HPV_vaccination_en.pdf [Accessed 2010 Nov 9]
Goldhaber-Fiebert JD, Stout NK, Salomon JA, et al. Cost-effectiveness of cervical cancer screening with human papillomavirus DNA testing and HPV-16,18 vaccination. J Natl Cancer Inst 2008; 100: 308–20
Mandelblatt JS, Lawrence WF, Womack SM, et al. Benefits and costs of using HPV testing to screen for cervical cancer. JAMA 2002; 287: 2372–81
Habbema JD, van Oortmarssen GJ, Lubbe JT, et al. The MISCAN simulation program for the evaluation of screening for disease. Comput Methods Programs Biomed 1985; 20: 79–93
Myers ER, McCrory DC, Nanda K, et al. Mathematical model for the natural history of human papillomavirus infection and cervical carcinogenesis. Am J Epidemiol 2000; 151: 1158–71
Goldhaber-Fiebert JD, Stout NK, Ortendahl J, et al. Modeling human papillomavirus and cervical cancer in the United States for analyses of screening and vaccination. Popul Health Metr 2007; 5: 11
Suarez E, Smith JS, Bosch FX, et al. Cost-effectiveness of vaccination against cervical cancer: a multi-regional analysis assessing the impact of vaccine characteristics and alternative vaccination scenarios. Vaccine 2008; 26 Suppl. 5: F29–45
Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making 1993; 13: 322–38
Neilson A, Freiesleben de Blasio B. Economic evaluation of the human papillomavirus(HPV): vaccination in Norway. 2007 [online]. Available from URL: http://www.mendeley.com/research/economic-evaluation-human-papillomavirus-hpvvaccination-norway/ [Accessed 2012 Feb 20]
Lauer JA, Röhrich K, Wirth H, et al. PopMod: a longitudinal population model with two interacting disease states. Cost Eff Resour Alloc 2003; 1(1): 6
Elbasha EH, Dasbach EJ, Insinga RP. Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis 2007; 13: 28–41
Kim JJ, Andres-Beck B, Goldie SJ. The value of including boys in an HPV vaccination programme: a cost-effectiveness analysis in a low-resource setting. Br J Cancer 2007; 97: 1322–8
Elbasha EH, Dasbach EJ, Insinga RP. A multi-type HPV transmission model. Bull Math Biol 2008; 70: 2126–76
Taira AV, Neukermans CP, Sanders GD. Evaluating human papillomavirus vaccination programs. Emerg Infect Dis 2004; 10: 1915–23
Insinga RP, Dasbach EJ, Elbasha EH, et al. Cost-effectiveness of quadrivalent human papillomavirus (HPV) vaccination in Mexico: a transmission dynamic model-based evaluation. Vaccine 2007; 26: 128–39
Acknowledgements
No sources of funding were used to conduct this study or prepare this manuscript. The authors have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seto, K., Marra, F., Raymakers, A. et al. The Cost Effectiveness of Human Papillomavirus Vaccines. Drugs 72, 715–743 (2012). https://doi.org/10.2165/11599470-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11599470-000000000-00000