Abstract
Background: sponsored by the pharmaceutical industry is often assumed to be more likely to report favourable cost-effectiveness results.
Objective: To determine whether there was a relationship between the source of funding and the reporting of positive results.
Methods: We conducted a systematic review of the literature to identify economic evaluations of bisphosphonates for the treatment of osteoporosis. We extracted the source of funding, region of study, the journal name and impact factor, and all reported incremental cost-effectiveness ratios (ICERs). We identified which ICERs were under the thresholds of $US20 000, $US50 000 and $US100 000 per QALY. A quality score between 0 and 7 was also given to each of the studies. We used generalized estimating equations for the analysis.
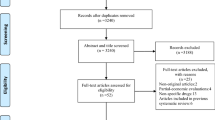
Result: The systematic review yielded 532 potential abstracts; 17 of these met our final eligibility criteria. Ten studies (59%) were funded by non-industry sources. A total of 571 ICERs were analysed. There was no significant difference between the number of industry- and non-industry-funded studies reporting ICERs below the thresholds of $US20 000 and $US50 000. However, industry-sponsored studies were more likely to report ICERs below $US100 000 (odds ratio = 4.69, 95% CI 1.77, 12.43). Studies of higher methodological quality (scoring >4.5 of 7) were less likely to report ICERs below $US20 000 and $US50 000 than studies of lower methodological quality (scores <4). Methodological quality was not significantly different between studies reporting ICERs under $US100 000.
Conclusions: In this relatively small sample of studies of bisphosphonates, the funding source (industry vs non-industry) did not seem to significantly affect the reporting of ICERs below the $US20 000 and $US50 000 thresholds. We hypothesize that methodological quality might be a more significant factor than the source of funding in differentiating which studies are likely to report favourable ICERs, with the higher-quality studies significantly less likely to report ICERs below $US20 000 and $US50 000 per QALY. Further research should explore this finding.







Similar content being viewed by others
References
Taylor RS, Drummond MF, Salkeld G, et al. Inclusion of cost effectiveness in licensing requirements of new drugs: the fourth hurdle. BMJ 2004 Oct 23; 329 (7472): 972–5
National Institute for Health and Clinical Excellence [online]. Guide to the methods of technology appraisal. London: NICE, 2008 Jun
Academy of Managed Care Pharmacy [online]. Available from URL: http://www.fmcpnet.org/data/resource/Format~Version_2_1~Final_Final.pdf [Accessed 2009 Dec 16]
Neumann PJ. Evidence-based and value-based formulary guidelines. Health Aff (Millwood) 2004 Jan-Feb; 23 (1): 124–34
Tufts Medical Center. The CEA registry [online]. Available from URL: https://research.tufts-nemc.org/cear/default.aspx [Accessed 2009 Dec 15]
Bell CM, Urbach DR, Ray JG, et al. Bias in published cost effectiveness studies: systematic review. BMJ 2006 Mar 25; 332 (7543): 699–703
Fleurence RL, Iglesias CP, Johnson JM. The cost effectiveness of bisphosphonates for the prevention and treatment of osteoporosis: a structured review of the literature. Pharmacoeconomics 2007; 25 (11): 913–33
Eichler HG, Kong SX, Gerth WC, et al. Use of costeffectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value Health 2004 Sep-Oct; 7 (5): 518–28
Evans C, Tavakoli M, Crawford B. Use of quality adjusted life years and life years gained as benchmarks in economic evaluations: a critical appraisal. Health Care Manag Sci 2004 Feb; 7 (1): 43–9
Laupacis A, Feeny D, Detsky AS, et al. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ 1992 Feb 15; 146 (4): 473–81
Neumann PJ, Greenberg D, Olchanski NV, et al. Growth and quality of the cost-utility literature, 1976–2001. Value Health 2005 Jan-Feb; 8 (1): 3–9
Owens DK. Interpretation of cost-effectiveness analyses. J Gen Intern Med 1998 Oct; 13 (10): 716–7
Neumann PJ, Stone PW, Chapman RH, et al. The quality of reporting in published cost-utility analyses, 1976–1997. Ann Intern Med 2000 Jun 20; 132 (12): 964–72
Ankjaer-Jensen A, Johnell O. Prevention of osteoporosis: cost-effectiveness of different pharmaceurical treatments. Osteoporos Int 1996; 6 (4): 265–75
Aursnes I, Storvik G, Gasemyr J, et al. A Bayesian analysis of bisphosphonate effects and cost-effectiveness in postmenopausal osteoporosis. Pharmacoepidemiol Drug Saf 2000; 9 (6): 501–9
Buckley LM, Hillner BE. Acost effectiveness analysis of calcium and vitamin D supplementation, etidronate, and alendronate in the prevention of vertebral fractures in women treated with glucocorticoids. J Rheumatol 2003 Jan; 30 (1): 132–8
Francis RM, Anderson FH, Torgerson DJ. A comparison of the effectiveness and cost of treatment for vertebral fractures in women. Br J Rheumatol 1995 Dec; 34 (12): 1167–71
Rodriguez Escolar C, Fidalgo Garcia ML, Rubio Cerbrian S. A cost-effectiveness analysis of alendronate compared to placebo in the prevention of hip fracture. Atencion Primaria 1999 Oct; 24 (7): 390–6
Rosner AJ, Grima DT, Torrance GW, et al. Cost effectiveness of multi-therapy treatment strategies in the prevention of vertebral fractures in postmenopausal women with osteoporosis. Pharmacoeconomics 1998 Nov; 14 (5): 559–73
Borgstrom F, Johnell O, Jonsson B, et al. Cost effectiveness of alendronate for the treatment of male osteoporosis in Sweden. Bone 2004 Jun; 34 (6): 1064–71
Brown MA, Bradlow J, Gray AM. Cost effectiveness of bone density measurements. J Br Menopause Soc 2001 Sep; 7 (3): 130–5
Christensen PM, Brixen K, Gyrd-Hansen D, et al. Costeffectiveness of alendronate in the prevention of osteoporotic fractures in Danish women. Basic Clin Pharmacol Toxicol 2005 May; 96 (5): 387–96
Coyle D, Cranney A, Lee KM, et al. Cost effectiveness of nasal calcitonin in postmenopausal women: use of Cochrane Collaboration methods for meta-analysis within economic evaluation. Pharmacoeconomics 2001; 19 (5 Pt 2): 565–75
Grima DT, Burge RT, Becker DL, et al. Short-term cost-effectiveness of bisphosphonate therapies for postmenopausal osteoporotic women at high risk of fracture. P T 2002 Sep; 27 (9): 448–55
Hart WM, Rubio-Terres C, Burrell A, et al. Pharmacoeconomic analysis of the treatment of postmenopausal osteoporosis with risedronate or alendronate. Rev Esp Enferm Metab Oseas 2002; 11 (3): 97–104
Iglesias CP, Torgerson DJ, Bearne A, et al. The cost utility of bisphosphonate treatment in established osteoporosis. QJM 2002 May; 95 (5): 305–11
Johnell O, Jonsson B, Jonsson L, et al. Cost effectiveness of alendronate (fosamax) for the treatment of osteoporosis and prevention of fractures. Pharmacoeconomics 2003; 21 (5): 305–14
Kanis JA, Borgstrom F, Johnell O, et al. Cost-effectiveness of risedronate for the treatment of osteoporosis and prevention of fractures in postmenopausal women. Osteoporos Int 2004 Nov; 15 (11): 862–71
Kanis JA, Brazier JE, Stevenson M, et al. Treatment of established osteoporosis: a systematic review and cost-utility analysis. Health Technol Assess 2002; 6 (29): 1–146
Mobley LR, Hoerger TJ, Wittenborn JS, et al. Costeffectiveness of osteoporosis screening and treatment with hormone replacement therapy, raloxifene, or alendronate. Med Decis Making 2006 Mar-Apr; 26 (2): 194–206
Pfister AK, Welch CA, Lester MD, et al. Cost-effectiveness strategies to treat osteoporosis in elderly women. South Med J 2006 Feb; 99 (2): 123–31
Schousboe JT, Ensrud KE, Nyman JA, et al. Potential cost-effective use of spine radiographs to detect vertebral deformity and select osteopenic post-menopausal women for amino-bisphosphonate therapy. Osteoporos Int 2005 Dec; 16 (12): 1883–93
Schousboe JT, Ensrud KE, Nyman JA, et al. Universal bone densitometry screening combined with alendronate therapy for those diagnosed with osteoporosis is highly cost-effective for elderly women. J Am Geriatr Soc 2005 Oct; 53 (10): 1697–704
Schousboe JT, Nyman JA, Kane RL, et al. Cost-effectiveness of alendronate therapy for osteopenic postmenopausal women. Ann Intern Med 2005 May 3; 142 (9): 734–41
Solomon DH, Kuntz KM. Should postmenopausal women with rheumatoid arthritis who are starting corticosteroid treatment be screened for osteoporosis? A cost-effectiveness analysis. Arthritis Rheum 2000 Sep; 43 (9): 1967–75
Stevenson M, Jones ML, De Nigris E, et al. A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis. Health Technol Assess 2005 Jun; 9 (22): 1–160
Hartmann M, Knoth H, Schulz D, et al. Industry-sponsored economic studies in oncology vs studies sponsored by nonprofit organisations. Br J Cancer 2003 Oct 20; 89 (8): 1405–8
Friedberg M, Saffran B, Stinson TJ, et al. Evaluation of conflict of interest in economic analyses of new drugs used in oncology. JAMA 1999 Oct 20; 282 (15): 1453–7
Hartmann M, Knoth H, Schulz D, et al. Industry-sponsored economic studies in critical and intensive care versus studies sponsored by nonprofit organizations. J Intensive Care Med 2003 Sep-Oct; 18 (5): 265–8
Baker CB, Johnsrud MT, Crismon ML, et al. Quantitative analysis of sponsorship bias in economic studies of antidepressants. Br J Psychiatry 2003 Dec; 183: 498–506
Miners AH, Garau M, Fidan D, et al. Comparing estimates of cost effectiveness submitted to the National Institute for Clinical Excellence (NICE) by different organisations: retrospective study. BMJ 2005 Jan 8; 330 (7482): 65
Braithwaite RS, Meltzer DO, King Jr JT, et al. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care 2008 Apr; 46 (4): 349–56
Luce BR, Mauskopf J, Sloan FA, et al. The return on investment in health care: from 1980 to 2000. Value Health 2006 May-Jun; 9 (3): 146–56
Coyle D, Tosteson AN. Towards a reference case for economic evaluation of osteoporosis treatments. J Rheumatol Suppl 2003 Dec; 68: 31–6
Sculpher M, Fenwick E, Claxton K. Assessing quality in decision analytic cost-effectiveness models: a suggested framework and example of application. Pharmacoeconomics 2000 May; 17 (5): 461–77
Weinstein MC, O’Brien B, Hornberger J, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices — Modeling Studies. Value Health 2003 Jan-Feb; 6 (1): 9–17
Lipsitz SR, Fitzmaurice GM, Orav EJ, et al. Performance of generalized estimating equations in practical situations. Biometrics 1994 Mar; 50 (1): 270–8
Acknowledgements
United BioSource Corporation is a private research organization that consults for several of the companies manufacturing the agents reviewed in this study. These companies were not involved in the funding, conception, development and writing of this article. There was no funding support received for this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fleurence, R.L., Spackman, D.E. & Hollenbeak, C. Does the Funding Source Influence the Results in Economic Evaluations?. Pharmacoeconomics 28, 295–306 (2010). https://doi.org/10.2165/11530530-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11530530-000000000-00000