Summary
Abstract
Orally administered lanthanum carbonate (Fosrenol®) dissociates in the acid environment of the upper gastrointestinal tract to release the cation lanthanum, which then binds dietary phosphate.
Lanthanum carbonate was effective in reducing levels of serum phosphate and serum calcium × phosphate product and then maintaining these levels within target ranges for up to 6 years in adult patients with end-stage renal disease (ESRD) on haemodialysis or peritoneal dialysis. The reduction in serum phosphate levels with lanthanum carbonate was generally similar to that with calcium carbonate or sevelamer hydrochloride. This agent was generally well tolerated, with the most frequently reported adverse events being gastrointestinal in nature and occurring at a similar rate to that with calcium carbonate. However, lanthanum carbonate was associated with fewer episodes of hypercalcaemia than calcium carbonate. Overall, lanthanum carbonate is a valuable option for the reduction of serum phosphate levels in patients with ESRD on haemodialysis or peritoneal dialysis.
Pharmacological Properties
Lanthanum carbonate dissociates in the acid environment of the upper gastrointestinal tract to release the trivalent cation lanthanum, which binds with high affinity to dietary phosphate in the stomach and upper small intestine, forming insoluble lanthanum phosphate.
Lanthanum carbonate reduces daily phosphate absorption. In patients with chronic kidney disease with residual kidney function, the decrease in urinary phosphate excretion (≈300mg/day) achieved with lanthanum carbonate ≤3000mg/day was estimated to be equivalent to about one-third of the daily phosphate absorption.
Lanthanum carbonate was not associated with detrimental effects on bone-cell activity, according to paired-bone biopsy studies of up to 2 years duration in patients with ESRD. With lanthanum carbonate versus comparator phosphate binder therapy, the percentage of patients with improved bone turnover after 1 year, but not 2 years, was significantly higher, and the percentage of patients with reduced bone volume was significantly lower after 2 years.
Lanthanum does not appear to cross the blood-brain barrier, according to data from animal studies. In patients with ESRD undergoing haemodialysis, there was no significant difference between lanthanum carbonate and alternative phosphate binder therapy in the decline in cognitive function that occurred over a 2-year period.
Systemic absorption of lanthanum carbonate is minimal (absolute bioavailability <0.002%). In patients with ESRD administered lanthanum carbonate 3000 mg/day for 10 days, the mean maximum plasma concentration of lanthanum was 1.0 ng/mL. Plasma concentrations of lanthanum did not increase with long-term (up to 6 years) administration of lanthanum carbonate. Bone biopsies taken from patients with ESRD who were treated with lanthanum carbonate for up to 4.5 years indicated an increase in bone lanthanum concentrations over the treatment phase (maximum concentration in any individual patient ≈10μg/g wet weight bone). After treatment cessation, lanthanum loss from bone was estimated to be ≈13% per year. In long-term animal studies, lanthanum concentrations increased over time in several tissues, including the liver; however, there was no evidence of adverse effects of this agent on the liver in clinical studies in which patients with ESRD received up to 6 years of lanthanum carbonate treatment.
Lanthanum is not metabolized and is not a substrate of cytochrome P450 enzymes. The mean elimination half-life was 52 hours in patients with ESRD. Lanthanum carbonate is mainly excreted via the biliary route.
Therapeutic Efficacy
In short-term, randomized, double-blind, multicentre trials in patients with ESRD on stable maintenance haemodialysis or peritoneal dialysis, lanthanum carbonate ≤3000 mg/day was effective in reducing serum phosphate and calcium × phosphateproduct levels and then maintaining these levels at target, while an increase in both these measures occurred with placebo. In a short-term, randomized, double-blind, multicentre trial in Japanese patients with ESRD in which phosphate binder dosages were titrated to achieve target serum phosphate levels, lanthanum carbonate 750–2250 mg/day was noninferior to calcium carbonate 1500–4500 mg/day in reducing serum phosphate levels. In another short-term, fixed-dosage, randomized, open-label, crossover trial, lanthanum carbonate 2250–3000 mg/day was not significantly different to sevelamer hydrochloride 4800–6400 mg/day in reducing serum phosphate levels in patients with ESRD on stable maintenance dialysis, according to an analysis of the intent-to-treat population (primary analysis). If the completer population of this study was analysed, then lanthanum carbonate was more effective than sevelamer hydrochloride.
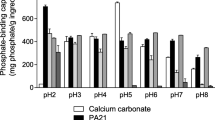
In randomized, open-label trials in adult patients with ESRD receiving maintenance haemodialysis, lanthanum carbonate 375–3000 mg/day was as effective in controlling serum levels of phosphate and calcium × phosphate product as conventional phosphate binders in a 24-month study and as calcium carbonate in 6-and 12-month studies. Extension studies demonstrated that control of serum phosphate and serum calcium × phosphate product levels was maintained for up to 6 years with lanthanum carbonate.
Tolerability
Lanthanum carbonate was generally well tolerated in short- and long-term clinical studies, with the most common adverse events being gastrointestinal (e.g. nausea, vomiting, diarrhoea, abdominal pain and constipation) and occurring with a similar incidence to that with other phosphate binders (including sevelamer hydrochloride and calcium-based binders). These adverse events were minimized by taking lanthanum carbonate with food, and generally abated over time with continued administration. The incidence of treatment-related adverse events did not increase with increased exposure to lanthanum carbonate, with no new or unexpected adverse events being reported in an extension study in which patients with ESRD received up to 6 years of treatment with this agent. Lanthanum carbonate was associated with fewer episodes of hypercalcaemia than calcium carbonate. With up to 6 years of lanthanum carbonate treatment, the incidence of fractures was low (4.3%).








Similar content being viewed by others
References
Burke SK. Phosphate is a uremic toxin. J Ren Nut 2008 Jan; 18(1): 27–32
Albaaj F, Hutchison A. Hyperphosphataemia in renal failure: causes, consequences and current management. Drugs 2003; 63(6): 577–96
Block GA, Hulbert-Shearon TE, Levin NW, et al. Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 1998 Apr; 31(4): 607–17
Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004 Aug; 15(4): 2208–18
Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2008 Sep; 52(3): 519–30
National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003 Oct; 42 (4 Suppl. 3): S1–201
Ashfaq A, Gitman M, Singhal PC, et al. Emerging strategies for lowering serum phosphorous in patients with end-stage renal disease. Expert Opin Pharmacother 2006; 7(14): 1897–905
Shire US Inc. Fosrenol® (lanthanum carbonate): US prescribing information [online]. Available from URL: http://www.fosrenol.com/PDFs/PrescribingInfo.pdf [Accessed 2009 May 10]
Shire Pharmaceuticals Limited. Fosrenol® 250 mg, 500 mg, 750mg and 1000mg chewable tablets: summary of product characteristics [online]. Available from URL: http://emc.medicines.org.uk/document.aspx?documentid=19617 [Accessed 2009 May 1]
Swainston Harrison T, Scott LJ. Lanthanum carbonate. Drugs 2004; 64(9): 985–96
Autissier V, Damment SJ, Henderson RA. Relative in vitro efficacy of the phosphate binders lanthanum carbonate and sevelamer hydrochloride. J Pharm Sci 2007 Oct; 96(10): 2818–27
Sprague SM. A comparative review of the efficacy and safety of established phosphate binders: calcium, sevelamer, and lanthanum carbonate. Curr Med Res Opin 2007 Dec; 23(12): 3167–75
Sprague SM, Abboud H, Qiu P, et al. Lanthanum carbonate reduces phosphorus burden in patients with CKD stages 3 and 4: a randomized trial. Clin J Am Soc Nephrol 2009 Jan; 4(1): 175–85
D’Haese PC, Spasovski GB, Sikole A, et al. A multicenter study on the effects of lanthanum carbonate (Fosrenol™) and calcium carbonate on renal bone disease in dialysis patients. Kidney Int 2003 Jun; 63 Suppl. 85: S73–8
Spasovski GB, Sikole A, Gelev S, et al. Evolution of bone and plasma concentration of lanthanum in dialysis patients before, during 1 year of treatment with lanthanum carbonate and after 2 years of follow-up. Nephrol Dial Transplant 2006 Aug; 21(8): 2217–24
Freemont AJ, Hoyland JA, Denton J, on behalf of the Lanthanum Carbonate SPD 405-303 Study Group. The effects of lanthanum carbonate and calcium carbonate on bone abnormalities in patients with end-stage renal disease. Clin Nephrol 2005 Dec; 64(6): 428–37
Malluche HH, Siami GA, Swanepoel C, et al. Improvements in renal osteodystrophy in patients treated with lanthanum carbonate for two years. Clin Nephrol 2008 Oct; 70(4): 284–95
Hutchison AJ, Barnett ME, Krause R, et al. Long-term efficacy and safety profile of lanthanum carbonate: results for up to 6 years of treatment. Nephron Clin Pract 2008; 110(1): c15–23
Altmann P, Barnett ME, Finn WF, et al. Cognitive function in stage 5 chronic kidney disease patients on hemodialysis: no adverse effects of lanthanum carbonate compared with standard phosphate-binder therapy. Kidney Int 2007; 71(3): 252–9
Damment SJP, Pennick M. Clinical pharmacokinetics of the phosphate binder lanthanum carbonate. Clin Pharmacokinet 2008; 47(9): 553–63
Pennick M, Dennis K, Damment SJ. Absolute bioavailability and disposition of lanthanum in healthy human subjects administered lanthanum carbonate. J Clin Pharmacol 2006 Jul; 46(7): 738–46
Finn WF, on behalf of the SPD 405-307 Lanthanum Study Group. Lanthanum carbonate versus standard therapy for the treatment of hyperphosphatemia: safety and efficacy in chronic maintenance hemodialysis patients. Clin Nephrol 2006 Mar; 65(3): 191–202
Hutchison AJ, Barnett ME, Krause R, et al. Lanthanum carbonate treatment, for up to 6 years, is not associated with adverse effects on the liver in patients with chronic kidney disease stage 5 receiving hemodialysis. Clin Nephrol 2009 Mar; 71(3): 286–95
Damment SJ, Cox AG, Secker R. Dietary administration in rodent studies distorts the tissue deposition profile of lanthanum carbonate; brain deposition is a contamination artefact? Toxicol Lett 2009 Aug 10; 188(3): 223–9
Bronner F, Slepchenko BM, Pennick M, et al. A model of the kinetics of lanthanum in human bone, using data collected during the clinical development of the phosphate binder lanthanum carbonate. Clin Pharmacokinet 2008; 47(8): 543–52
How PP, Fischer JH, Arruda JA, et al. Effects of lanthanum carbonate on the absorption and oral bioavailability of ciprofloxacin. Clin J Am Soc Nephrol 2007 Nov; 2(6): 1235–40
Dammet S, Pennick M, Dennis K. A new 1000-mg formulation of lanthanum carbonate effectively reduces urinary phosphorus excretion in healthy volunteers [abstract no. F-PO982]. J Am Soc Nephrol 2005; 16: 550A
Finn WF, Joy MS, Hladik G. Efficacy and safety of lanthanum carbonate for reduction of serum phosphorus in patients with chronic renal failure receiving hemodialysis. Clin Nephrol 2004 Sep; 62(3): 193–201
Shigematsu T, and the Lanthanum Carbonate Research Group. Lanthanum carbonate effectively controls serum phosphate without affecting serum calcium levels in patients undergoing hemodialysis. Ther Apher Dial 2008 Feb; 12(1): 55–61
Al-Baaj F, Speake M, Hutchison AJ. Control of serum phosphate by oral lanthanum carbonate in patients undergoing haemodialysis and continuous ambulatory peritoneal dialysis in a short-term, placebo-controlled study. Nephrol Dial Transplant 2005 Apr; 20(4): 775–82
Chiang SS, Chen JB, Yang WC. Lanthanum carbonate (Fosrenol®) efficacy and tolerability in the treatment of hyperphosphatemic patients with end-stage renal disease. Clin Nephrol 2005 Jun; 63(6): 461–70
Joy MS, Finn WF, on behalf of the LAM-302 Study Group. Randomized, double-blind, placebo-controlled, dose-titration, phase III study assessing the efficacy and tolerability of lanthanum carbonate: a new phosphate binder for the treatment of hyperphosphatemia. Am J Kidney Dis 2003 Jul; 42(1): 96–107
Shigematsu T, and the Lanthanum Carbonate Group. Multicenter prospective randomized, double-blind comparative study between lanthanum carbonate and calcium carbonate as phosphate binders in Japanese hemodialysis patients with hyperphosphatemia. Clin Nephrol 2008 Nov; 70(5): 404–10
Sprague SM, Ross EA, Nath SD, et al. Lanthanum carbonate vs. sevelamer hydrochloride for the reduction of serum phosphorus in hemodialysis patients: a crossover study. Clin Nephrol 2009 Oct; 72(4): 252–8
Hutchison AJ, Maes B, Vanwalleghem J, et al. Efficacy, tolerability, and safety of lanthanum carbonate in hyperphosphatemia: a 6-month, randomized, comparative trial versus calcium carbonate. Nephron Clin Pract 2005; 100(1): c8–19
Finn WF, Joy MS, on behalf of the LAM-308 Study Group. A long-term, open-label extension study on the safety of treatment with lanthanum carbonate, a new phosphate binder, in patients receiving hemodialysis. Curr Med Res Opin 2005 May; 21(5): 657–64
Hutchison AJ, Maes B, Vanwalleghem J, et al. Long-term efficacy and tolerability of lanthanum carbonate: results from a 3-year study. Nephron Clin Pract 2006; 102(2): c61–71
US Department of Health and Human Services — Food and Drug Administration. Statistical review and evaluation: clinical studies. Available from URL: http://www.fda.gov/cder/foi/nda/2004/21-468_Fosrenol_StatrClinStudy.pdf [Accessed 2009 Apr 1]
Hutchison AJ, Speake M, Al-Baaj F. Reducing high phosphate levels in patients with chronic renal failure undergoing dialysis: a 4-week, dose-finding, open-label study with lanthanum carbonate. Nephrol Dial Transplant 2004 Jul; 19(7): 1902–6
Eknoyan G, Levin A, Levin NW, et al. Bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003; 42 (4 Suppl. 3): 1–201
Hutchison AJ. Oral phosphate binders. Kidney Int 2009 May; 75(9): 906–14
Shire Plc. Shire announces launch of FOSRENOL® in Japan: management of hyperphosphataemia in end-stage renal disease patients [media release]. Available from URL: http://www.medicalnewstoday.com/articles/142033.php [Accessed 2009 Mar 12]
Hruska KA, Mathew S, Lund R, et al. Hyperphosphatemia of chronic kidney disease. Kidney Int 2008; 74(2): 148–57
Ritz E. The clinical management of hyperphosphatemia. J Nephrol 2005; 18(3): 221–8
Moe SM, Drüeke TB, Block GA, et al. KDIGO clinical practice guidelines for the prevention, diagnosis, evaluation, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 2009 Aug; (113): S1-130
Kanbay M, Goldsmith D, Akcay Al, et al. Phosphate — the silent stealthy cardiorenal culprit in all stages of chronic kidney disease: a systematic review. Blood Purif 2009; 27: 220–30
Kestenbaum B, Sampson JN, Rudser KD, et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 2005 Feb; 16(2): 520–8
Voormolen N, Noordzij M, Grootendorst DC, et al. High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol Dial Transplant 2007 Oct; 22(10): 2909–16
Jindal K, Chan CT, Deziel C, et al. Hemodialysis clinical practice guidelines for the Canadian Society of Nephrology. J Am Soc Nephrol 2006; 17 (3 Suppl. 1): S1–27
Levin A, Hemmelgarn B, Culleton B, et al. Guidelines for the management of chronic kidney disease. CMAJ 2008 Nov 18; 179(11): 1154–62
Joint Speciality Committee on Renal Medicine of the Royal College of Physicians and the Renal Association. Chronic kidney disease in adults: UK guidelines for identification, management and referral [online]. Available from URL: http://www.renal.org/CKDguide/full/UKCKDfull.pdf [Accessed 2009 Apr 22]
Elder G, Faull R, Branley P, et al. Management of bone disease, calcium, phosphate and parathyroid hormone. Nephrology 2006; 11 Suppl.: S230–61
Mohammed IA, Hutchison AJ. Phosphate binding therapy in dialysis patients: focus on lanthanum carbonate. Ther Clin Risk Manag 2008 Oct; 4(5): 887–93
Shinaberger CS, Greenland S, Kopple JD, et al. Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am J Clin Nutr 2008 Dec; 88(6): 1511–8
Pauly RP. Nocturnal home hemodialysis and short daily hemodialysis compared with kidney transplantation: emerging data in a new era. Adv Chronic Kidney Dis 2009 May; 16(3): 169–72
Mucsi I, Hercz G, Uldall R, et al. Control of serum phosphate without any phosphate binders in patients treated with nocturnal hemodialysis. Kidney Int 1998; 53(5): 1399–401
Buoncristiani U. Fifteen years of clinical experience with daily haemodialysis. Nephrol Dial Transplant 1998; 13 Suppl. 6: 148–51
Mohammed I, Hutchison AJ. Oral phosphate binders for the management of serum phosphate levels in dialysis patients. J Ren Care 2009 Mar; 35 Suppl. 1: 65–70
Wills MR, Savory J. Aluminium poisoning: dialysis encephalopathy, osteomalacia and anaemia. Lancet 1983 Jul 2; II(8340): 29–34
Gonzalez-Revalderia J, Casares M, de Paula M, et al. Biochemical and haematological changes in low-level aluminium intoxication. Clin Chem Lab Med 2000 Mar; 38(3): 221–5
Schaefer K, Umlauf E, von Herrath D. Reduced risk of hypercalcemia for hemodialysis patients by administering calcitriol at night. Am J Kidney Dis 1992 May; 19(5): 460–4
Goodman WG. Vascular calcification in chronic renal failure. Lancet 2001 Oct; 358(9288): 1115–6
Locatelli F, Cannata-Andia JB, Drüeke TB, et al. Management of disturbances of calcium and phosphate metabolism in chronic renal insufficiency, with emphasis on the control of hyperphosphataemia. Nephrol Dial Transplant 2002 May; 17(5): 723–31
O’Donovan R, Baldwin D, Hammer M, et al. Substitution of aluminium salts by magnesium salts in control of dialysis hyperphosphataemia. Lancet 1986 Apr; I(8486): 880–2
Spiegel DM. The role of magnesium binders in chronic kidney disease. Semin Dial 2007; 20(4): 333–6
Goldsmith DR, Scott LJ, Cvetkovic RS, et al. Sevelamer hydrochloride: a review of its use for hyperphosphataemia in patients with end-stage renal disease on haemodialysis. Drugs 2008; 68(1): 85–104
Delmez J, Block G, Robertson J, et al. A randomized, double-blind, crossover design study of sevelamer hydrochloride and sevelamer carbonate in patients on hemodialysis. Clin Nephrol 2007 Dec; 68(6): 386–91
Chertow G, Dillon M, Burke S, et al. A randomized trial of sevelamer hydrochloride (Renagel) with and without supplemental calcium: strategies for the control of hyperphosphataemia and hyperparathyroidism in hemodialysis patients. Clin Nephrol 1999 Jan; 51(1): 18–26
Karamanidou C, Clatworthy J, Weinman J, et al. A systematic review of the prevalence and determinants of non-adherence to phosphate binding medication in patients with end-stage renal disease. BMC Nephrol 2008 Jan 31; 9 (2)
Tomasello S, Dhupar S, Sherman R. Phosphate binders, K/DOQI guidelines, and compliance: the unfortunate reality. Dialysis Transplant 2004; 33: 236–42
US Renal Data System. Medication use among dialysis patients in the Dialysis and Morbidity and Mortality Study. Am J Kidney Dis 1998; 32 (2 Suppl. 1): S60–8
Hutchison AJ, Laville M, SPD405-313 Lanthanum Study Group. Switching to lanthanum carbonate monotherapy provides effective phosphate control with a low tablet burden. Nephrol Dial Transplant 2008 Nov; 23(11): 3677–84
Mehrotra R, Martin KJ, Fishbane S, et al. Higher strength lanthanum carbonate provides serum phosphorus control with a low tablet burden and is preferred by patients and physicians: a multicenter study. Clin J Am Soc Nephrol 2008 Sep; 3(5): 1437–45
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: M. Emmett, Department of Internal Medicine, Baylor University Medical Center, Dallas, Texas, USA; D.J. Goldsmith, Renal Unit, Guy’s Hospital and St Thomas’ Hospital, London, UK; A.J. Hutchison, Manchester Royal Infirmary, Manchester, UK; K.J. Martin, Department of Internal Medicine, Saint Louis University, St Louis, Missouri, USA; R. Mehrotra, Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, California, USA; G.A. Siami, Department of Medicine, Division of Nephrology, Vanderbilt University Medical Center, Nashville, Tennessee, USA.
Data Selection
Sources: Medical literature published in any language since 1980 on ‘lanthanum carbonate’, identified using MEDLINE and EMBASE, supplemented by AdisBase (a proprietary database). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: MEDLINE, EMBASE and AdisBase (a proprietary database) search terms were ‘lanthanum carbonate’ and ‘hyperphosphataemia’. Searches were last updated 15 October 2009.
Selection: Studies in patients with end-stage renal disease who received lanthanum carbonate. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Lanthanum carbonate, end-stage renal disease, hyperphosphataemia, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability.
Rights and permissions
About this article
Cite this article
Curran, M.P., Robinson, D.M. Lanthanum Carbonate. Drugs 69, 2329–2349 (2009). https://doi.org/10.2165/11202610-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11202610-000000000-00000