Abstract
Background
Management of neuroendocrine tumor liver metastasis (NELM) remains controversial, with some advocating an aggressive surgical approach while others have adopted a more conservative strategy. We sought to define the efficacy of the surgical management of NELM in a large multicenter international cohort of patients.
Methods
We identified 339 patients who underwent surgical management for NELM from 1985 to 2009 from an international database of eight major hepatobiliary centers. Relevant clinicopathologic data were assessed using Kaplan–Meier and Cox regression models.
Results
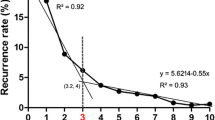
Most patients had a pancreatic (40%) or small bowel (25%) neuroendocrine tumor (NET) primary. The majority of patients (60%) had bilateral liver disease. At surgery, 78% of patients underwent hepatic resection, 3% ablation alone, and 19% resection + ablation. Major hepatectomy was performed in 45% of patients, and 14% underwent a second liver operation. Carcinoid was the most common NET histological subtype (53%). Median survival was 125 months, with overall 5- and 10-year survival of 74%, and 51%, respectively. Disease recurred in 94% of patients at 5 years. Patients with hormonally functional NET who had R0/R1 resection benefited the most from surgery (P = 0.01). On multivariate analyses, synchronous disease [hazard ratio (HR) = 1.9], nonfunctional NET hormonal status (HR = 2.0), and extrahepatic disease (HR = 3.0) remained predictive of worse survival (all P < 0.05).
Conclusions
Liver-directed surgery for NELM is associated with prolonged survival; however, the majority of patients will develop recurrent disease. Patients with hormonally functional hepatic metastasis without prior extrahepatic or synchronous disease derive the greatest survival benefit from surgical management.



Similar content being viewed by others
References
Chamberlain RS, Canes D, Brown KT, et al. Hepatic neuroendocrine metastases: does intervention alter outcomes? J Am Coll Surg. 2000;190(4):432–45.
Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97(4):934–59.
Thompson GB, van Heerden JA, Grant CS, et al. Islet cell carcinomas of the pancreas: a twenty-year experience. Surgery. 1988;104(6):1011–7.
Godwin JD II. Carcinoid tumors. An analysis of 2,837 cases. Cancer. 1975;36(2):560–9.
Chen H, Hardacre JM, Uzar A, et al. Isolated liver metastases from neuroendocrine tumors: does resection prolong survival? J Am Coll Surg. 1998;187(1):88–92 (discussion 92–3).
Sarmiento JM, Heywood G, Rubin J, et al. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg. 2003;197(1):29–37.
Knox CD, Anderson CD, Lamps LW, et al. Long-term survival after resection for primary hepatic carcinoid tumor. Ann Surg Oncol. 2003;10(10):1171–5.
Nave H, Mossinger E, Feist H, et al. Surgery as primary treatment in patients with liver metastases from carcinoid tumors: a retrospective, unicentric study over 13 years. Surgery. 2001;129(2):170–5.
Touzios JG, Kiely JM, Pitt SC, et al. Neuroendocrine hepatic metastases: does aggressive management improve survival? Ann Surg. 2005;241(5):776–83 (discussion 783–5).
Scigliano S, Lebtahi R, Maire F, et al. Clinical and imaging follow-up after exhaustive liver resection of endocrine metastases: a 15-year monocentric experience. Endocr Relat Cancer. 2009;16(3):977–90.
Elias D, Lasser P, Ducreux M, et al. Liver resection (and associated extrahepatic resections) for metastatic well-differentiated endocrine tumors: a 15-year single center prospective study. Surgery. 2003;133(4):375–82.
Strasberg SM. The International Hepato-Pancreato-Biliary Association. The Brisbane 2000 terminology of liver anatomy and resections. HBP. 2000;2(3):333–9.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Cox D. Regression models and life tables. J R Stat Soc B. 1972;34:187–220.
Hosmer DW, Lemeshow S. Applied survival analysis: regression modeling of time to event data. 2nd ed. New York: Wiley; 1999.
May S, Hosmer DW. A simplified method of calculating an overall goodness-of-fit test for the Cox proportional hazards model. Lifetime Data Anal. 1998;4(2):109–20.
Que FG, Nagorney DM, Batts KP, et al. Hepatic resection for metastatic neuroendocrine carcinomas. Am J Surg. 1995;169(1):36–42 (discussion 42–3).
de Jong MC, Mayo SC, Pulitano C, et al. Repeat curative intent liver surgery is safe and effective for recurrent colorectal liver metastasis: results from an international multi-institutional analysis. J Gastrointest Surg. 2009;250(3):440–8.
de Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250(3):440–8.
Pawlik TM, Vauthey JN. Surgical margins during hepatic surgery for colorectal liver metastases: complete resection not millimeters defines outcome. Ann Surg Oncol. 2008;15(3):677–9.
Elias D, Lefevre JH, Duvillard P, et al. Hepatic metastases from neuroendocrine tumors with a “thin slice” pathological examination: they are many more than you think. Ann Surg. 2010;251(2):307–10.
Asiyanbola B, Chang D, Gleisner AL, et al. Operative mortality after hepatic resection: are literature-based rates broadly applicable? J Gastrointest Surg. 2008;12(5):842–51.
Pawlik TM, Izzo F, Cohen DS, et al. Combined resection and radiofrequency ablation for advanced hepatic malignancies: results in 172 patients. Ann Surg Oncol. 2003;10(9):1059–69.
Mazzaglia PJ, Berber E, Milas M, et al. Laparoscopic radiofrequency ablation of neuroendocrine liver metastases: a 10-year experience evaluating predictors of survival. Surgery. 2007;142(1):10–9.
Disclosures
Dr. Pawlik has served as a consultant to Bayer-Onyx. Dr. Choti is the Johns Hopkins University institutional principal investigator for clinical trials sponsored by Ipsen Corporation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mayo, S.C., de Jong, M.C., Pulitano, C. et al. Surgical Management of Hepatic Neuroendocrine Tumor Metastasis: Results from an International Multi-Institutional Analysis. Ann Surg Oncol 17, 3129–3136 (2010). https://doi.org/10.1245/s10434-010-1154-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-010-1154-5