-
PDF
- Split View
-
Views
-
Cite
Cite
Edgardo Somigliana, Fedro Alessandro Peccatori, Francesca Filippi, Fabio Martinelli, Francesco Raspagliesi, Ida Martinelli, Risk of thrombosis in women with malignancies undergoing ovarian stimulation for fertility preservation, Human Reproduction Update, Volume 20, Issue 6, November/December 2014, Pages 944–951, https://doi.org/10.1093/humupd/dmu035
Close - Share Icon Share
Compared with the general population, cancer patients have a higher risk of venous thromboembolism as well as arterial thrombotic events such as stroke, myocardial infarction and peripheral arterial embolism. Therefore a possible concern for women with malignancies undergoing ovarian stimulation for fertility preservation is the increased risk of venous or arterial thrombosis.
In this article, we revised current available literature on the risk of thrombosis in patients with cancer and in women undergoing ovarian stimulation, with the ultimate aim of drawing some indications for preventive measures.
Unfortunately, there are no specific data on the risk of thrombosis in women with cancer undergoing ovarian stimulation for fertility preservation. However, the literature suggests that the cancer type and stage, surgery, and chemotherapy all influence the risk of venous and, possibly, arterial thrombosis. Reports of cases of ovarian stimulation in women without malignancies have shown that venous thrombosis rarely occurs unless a pregnancy is achieved, while arterial thrombosis can occur in the absence of pregnancy but is usually only associated with ovarian hyperstimulation syndrome (OHSS). OHSS increases the risk of thrombotic events, but only the early form of the syndrome is relevant for women undergoing fertility preservation.
The available evidence on the risks of thrombosis for women undergoing ovarian stimulation for fertility preservation due to a malignancy is reassuring. However the avoidance of the early form of OHSS in women preserving oocytes/embryos due to malignancy is crucial. For these cycles, we advocate the use of a regimen of ovarian stimulation with gonadotrophin releasing hormone (GnRH) antagonists using GnRH agonists to trigger ovulation, an approach that has been shown to markedly reduce the risk of OHSS. Antithrombotic prophylaxis should be administered only to selected subgroups of women such as those with other risk factors or those who do develop early OHSS.
Introduction
There is a growing and widespread consensus that women of reproductive age who are at risk of ovarian failure due to cancer treatments should be offered fertility preservation (Loren et al., 2013). Cryostorage of ovarian fragments represents one of the most innovative and fascinating opportunities to preserve fertility and is the only possibility in pre-menarchal girls (Jadoul and Kim, 2012; Donnez et al., 2013). However, this option is still experimental and cryostorage of embryos or oocytes after ovarian stimulation remains the most common approach for adult women (Bedoschi and Oktay, 2013; Garcia-Velasco et al., 2013; Loren et al., 2013). Physicians engaged in reproductive medicine feel familiar and confident with the cryostorage of embryos and gametes and this has facilitated its diffusion. Moreover, recent findings have helped to overcome two of the main limitations of ovarian stimulation in women with malignancies, i.e. the possible interaction of high estrogen levels with hormonally sensitive cancer growth and the necessity to delay the initiation of cancer treatment. The concomitant use of letrozole during ovarian stimulation in women with hormonally sensitive cancers significantly reduces the estrogen peak (Cakmak and Rosen, 2013) and its use has been acknowledged in the recent guidelines of the American Society of Clinical Oncology (ASCO) (Loren et al., 2013). Additionally, ovarian stimulation can be initiated in any phase of the menstrual cycle (the ‘random-start’ approach), thus reducing the time required for obtaining oocytes or embryos to <2 weeks (Cakmak and Rosen, 2013; Cakmak et al., 2013).
Nonetheless, oocytes or embryos cryostorage for cancer patients is still viewed with scepticism by some physicians who fear the possible side effects of this procedure. In this review, we focused on the risk of venous and arterial thrombosis, neglected but potentially relevant complications of ovarian stimulation, that may be enhanced in women with malignancies. To address this, we discuss the relationship between cancer, thrombosis and ovarian stimulation and try to provide some clinical recommendations on prevention measures.
Methods
We searched PUBMED for articles published in the English language between September 1990 and March 2014 using the following MeSH search terms: ‘fertility preservation’ OR ‘Assisted Reproductive Technology’ combined with ‘cancer’ OR ‘malignancy’ OR ‘thrombosis’ OR ‘stroke’ OR ‘myocardial infarction’ OR ‘pulmonary embolism’ OR ‘thromboembolism’ with restriction to the human species. Data were extracted independently by four investigators (F.A.P., F.F., F.M. and F.R.) who also performed an initial screening of the title and abstract of all articles to exclude citations deemed irrelevant. Reference lists of the selected articles and from other reviews were also evaluated.
Malignancies and thrombosis
Cancer is one of the most relevant acquired risk factor for venous thromboembolism (VTE) which represents the second leading cause of cancer death and a major cause of morbidity, medical care and costs (Khorana et al., 2009; Khorana, 2010; Lyman et al., 2013). The estimated incidence of VTE in cancer patients is up to 10-fold higher than that in the general population (Blom et al., 2006; Khorana et al., 2013). A recent meta-analysis including 38 studies reported a mean annual VTE incidence of 1.4% (95% CI: 0.7–2.5%) among cancer patients (Horsted et al., 2012), thus markedly higher than the annual incidence of 0.02–0.2% observed in the general population (Lidegaard et al., 2012a; Mahmoodi et al., 2012; Khorana et al., 2013). Accordingly, ∼20% of idiopathic VTE cases occur in patients with cancer and the risk is higher in those with distant metastases (Caine et al., 2002; Blom et al., 2005).
The pathogenesis of thrombosis in cancer patients is complex. Cancer cells can promote the activation of blood coagulation directly by secreting cysteine proteases and tissue factors, or indirectly by stimulating endothelial cells and circulating mononuclear cells to release pro-coagulant factors (Bick, 2003; Previtali et al., 2011). Moreover, chemotherapy markedly increases the risk of thrombosis. The mechanisms underlying the pro-thrombotic effects of chemotherapy have been only partially elucidated, are presumably multifactorial and vary with the different drugs. The most thrombogenic chemotherapeutic agents are fluorouracil, cisplatin and paclitaxel (Khorana et al., 2005, 2013). A pro-thrombotic effect is recognized also for hormonal agents such as tamoxifene (Blom et al., 2005; Cuzick et al., 2007) and for anti-angiogenetic agents, including monoclonal antibodies and tyrosine kinase inhibitors (Zangari et al., 2009; Raschi and De Ponti, 2012). Finally, surgery (in particular extensive surgery) plays an important role in the overall increased risk of VTE in women with cancer (Lyman et al., 2013).
The annual reported VTE incidence rates in cancer patients vary widely among published reports, from 0.6% to >18% (Horsted et al., 2012). This difference can be explained by the characteristics of the populations investigated, the duration of follow-up, the definition of VTE and the relative frequency of the specific cancer types (Blom et al., 2005; Chew et al., 2008; Khorana et al., 2009, 2013; Wun and White, 2009). However it is likely that women undergoing ovarian stimulation for fertility preservation have a lower risk of VTE when compared with the general oncologic population because patients referred for such procedures are young and preferably have early-stage cancers. Moreover, the most common oncological diagnosis among women who undergo ovarian stimulation for fertility preservation is early breast cancer, a condition actually associated with a lower risk of VTE compared with other malignancies (Table I) (Wun and White, 2009; Klock et al., 2010; Barcroft et al., 2013; Garcia-Velasco et al., 2013; Johnson et al., 2013). Of note, the rates of VTE reported in Table I may appear high. However, these are yearly rates and they refer to an overall patient population, thus including older subjects, advanced cancers and patients undergoing surgery or receiving chemotherapy.
Most common diagnoses of malignancies in women preserving oocytes/embryos and corresponding risk of VTE.
| Diagnosis . | Frequency in oocyte-embryo banking programsa . | Annual rate of VTEb . |
|---|---|---|
| Breast cancer | 36–67% | 0.9% |
| Hodgkin's Lymphoma | 4–21% | 3.7% |
| Non-Hodgkin's Lymphoma | 2–5% | 3.7% |
| Leukemia | 4–13% | 1.8–7.4% |
| Cervical cancer | 7–12% | 1.7% |
| Ovarian cancer | 3–7% | 4.2% |
| Gastrointestinal tumours | 4% | 2.7–14.0% |
| Other cancer | 6–31% | 0.5–11.1% |
| Diagnosis . | Frequency in oocyte-embryo banking programsa . | Annual rate of VTEb . |
|---|---|---|
| Breast cancer | 36–67% | 0.9% |
| Hodgkin's Lymphoma | 4–21% | 3.7% |
| Non-Hodgkin's Lymphoma | 2–5% | 3.7% |
| Leukemia | 4–13% | 1.8–7.4% |
| Cervical cancer | 7–12% | 1.7% |
| Ovarian cancer | 3–7% | 4.2% |
| Gastrointestinal tumours | 4% | 2.7–14.0% |
| Other cancer | 6–31% | 0.5–11.1% |
VTE, venous thromboembolism.
aData from Klock et al. (2010), Barcroft et al. (2013), Johnson et al. (2013) and Garcia-Velasco et al. (2013).
bData are indicative and refer to a single large representative study (Wun and White, 2009).
Most common diagnoses of malignancies in women preserving oocytes/embryos and corresponding risk of VTE.
| Diagnosis . | Frequency in oocyte-embryo banking programsa . | Annual rate of VTEb . |
|---|---|---|
| Breast cancer | 36–67% | 0.9% |
| Hodgkin's Lymphoma | 4–21% | 3.7% |
| Non-Hodgkin's Lymphoma | 2–5% | 3.7% |
| Leukemia | 4–13% | 1.8–7.4% |
| Cervical cancer | 7–12% | 1.7% |
| Ovarian cancer | 3–7% | 4.2% |
| Gastrointestinal tumours | 4% | 2.7–14.0% |
| Other cancer | 6–31% | 0.5–11.1% |
| Diagnosis . | Frequency in oocyte-embryo banking programsa . | Annual rate of VTEb . |
|---|---|---|
| Breast cancer | 36–67% | 0.9% |
| Hodgkin's Lymphoma | 4–21% | 3.7% |
| Non-Hodgkin's Lymphoma | 2–5% | 3.7% |
| Leukemia | 4–13% | 1.8–7.4% |
| Cervical cancer | 7–12% | 1.7% |
| Ovarian cancer | 3–7% | 4.2% |
| Gastrointestinal tumours | 4% | 2.7–14.0% |
| Other cancer | 6–31% | 0.5–11.1% |
VTE, venous thromboembolism.
aData from Klock et al. (2010), Barcroft et al. (2013), Johnson et al. (2013) and Garcia-Velasco et al. (2013).
bData are indicative and refer to a single large representative study (Wun and White, 2009).
The relationship between cancer and arterial thrombotic events (ATE) such as stroke, myocardial infarction and peripheral arterial embolism has been less extensively investigated. The annual reported incidences of ATE in subjects with cancer vary between 1.5 and 5.5% (Khorana et al., 2006, 2008; Scappaticci et al., 2007), thus higher than the 0.03–1.8% annual incidence observed in the general population (Lidegaard et al., 2012b; Estruch et al., 2013; Yang et al., 2013). Patients with haematological malignancies such as acute leukaemia or myeloproliferative neoplasms have the highest risk (De Stefano et al., 2005; Khorana et al., 2006).
The pathogenesis of ATE partly differs compared with VTE. ATE in malignancies have often been described as secondary to tumour-cell emboli, emboli originating from non-bacterial thrombotic endocarditis and, in case of stroke, as paradoxical emboli from deep venous thrombosis passing through a patent foramen ovale at the base of the skull (Javid et al., 2008).
Within this complex scenario for VTE and ATE, the identification of subjects with cancer who may benefit from antithrombotic prophylaxis is challenging. The available guidelines and opinions on the prevention of VTE strengthen the importance of a case by case approach that takes into consideration all risk factors, either cancer-related (site, stage, histology and time after initial diagnosis), treatment-related (chemotherapy, anti-angiogenetic agents, hormonal therapy, erythropoiesis-stimulating agents, transfusion, indwelling venous access device, radiotherapy and surgery for >60 min) and patient related (age, race, medical comorbidities, obesity, history of VTE, thrombophilia or blood counts abnormalities) (Khorana et al., 2013; Lyman et al., 2013; Gomes and Khorana, 2014). The ASCO guidelines on the prevention of VTE in cancer patients state that antithrombotic prophylaxis is not routinely recommended. Low-molecular-weight heparin (LMWH) should be administered to hospitalized patients with cancer, but it is not routinely recommended for out-patients, with the exception of very selected high-risk subjects. Moreover, the ASCO guidelines state that patients undergoing major cancer surgery should receive prophylaxis before surgery and for at least 7–10 days. In those with high-risk features, post-operative prophylaxis may be extended to up to 4 weeks (Lyman et al., 2013). To our knowledge, there is no guideline for the prevention of ATE in subjects with malignancies.
Ovarian stimulation and thrombosis
A causal relationship between ovarian stimulation and thrombosis was firstly suggested in a case report published in The Lancet in 1965 (Mozes et al., 1965; ESHRE, 2013). Following this first case, a number of papers have reported this complication and >100 cases have been published (Stewart et al., 1997; Rao et al., 2005; Chan and Ginsberg, 2006; Chan and Dixon, 2008; Chan, 2009; Chipwete et al., 2009; Nelson, 2009; Seong et al., 2010; Dorais et al., 2011; Fleming et al., 2012; Mmbaga et al., 2012; Meshksar et al., 2013). In recent years, some reviews of the literature have tried to draw some information from a global analysis of all these reported cases (Stewart et al., 1997; Rao et al., 2005; Chan and Ginsberg, 2006; Chan and Dixon, 2008; Chan, 2009; Nelson, 2009). Although the information obtained from these analyses should be considered with caution because of possible confounders and publication biases, some interesting insights have emerged (Table II). Firstly, both arterial and venous events can occur after ovarian stimulation. Secondly, the occurrence of pregnancy may play a significant role in VTE but not in ATE. Indeed, VTE almost invariably occurred in women achieving a pregnancy. In contrast, ATEs have been recorded earlier and they can also occur if pregnancy is not achieved. Thirdly, ovarian hyperstimulation syndrome (OHSS), a complication of ovarian stimulation characterized by enlarged ovaries, fluid retention and haemoconcentration, plays a crucial for both VTE and ATE. On these bases, the risk of either VTE and ATE is presumably low, especially if cycles are not complicated by OHSS. Finally, the majority of the events have occurred in women without thrombophilic disorders. Of note, the proportion of women with thrombophilia in women with VTE and ATE was 19–26% and the 41–48%, respectively.
Main insights from the published case reports on the relationship between ovarian hyper-stimulation and thrombosis.
| Characteristics . | ATE . | VTE . |
|---|---|---|
| Relative frequency (ATE versus VTE) | 1 | 2 |
| Concomitant to OHSS | 90–95% | 70–78% |
| Before final oocyte maturation trigger | 3% | 3% |
| Concomitant to pregnancy | 46% | 97% |
| Mean time since embryo transfer (days) | 10–11 | 40–42 |
| Thrombophyliaa | 19–26% | 41–48% |
| Most common location/ | Brain (stroke), extremities, heart (myocardial infarction) | Upper extremities, neck, intracranial |
| Characteristics . | ATE . | VTE . |
|---|---|---|
| Relative frequency (ATE versus VTE) | 1 | 2 |
| Concomitant to OHSS | 90–95% | 70–78% |
| Before final oocyte maturation trigger | 3% | 3% |
| Concomitant to pregnancy | 46% | 97% |
| Mean time since embryo transfer (days) | 10–11 | 40–42 |
| Thrombophyliaa | 19–26% | 41–48% |
| Most common location/ | Brain (stroke), extremities, heart (myocardial infarction) | Upper extremities, neck, intracranial |
Adapted from Stewart et al. (1997), Rao et al. (2005), Chan and Ginsberg (2006), Chan and Dixon (2008), Chan (2009) and Nelson (2009).
ATE, arterial thrombotic events; VTE, venous thromboembolism; OHSS, ovarian hyperstimulation.
aIncludes women heterozygous for methylene-tetra-hydro-folate-reductase (MTHFR).
Main insights from the published case reports on the relationship between ovarian hyper-stimulation and thrombosis.
| Characteristics . | ATE . | VTE . |
|---|---|---|
| Relative frequency (ATE versus VTE) | 1 | 2 |
| Concomitant to OHSS | 90–95% | 70–78% |
| Before final oocyte maturation trigger | 3% | 3% |
| Concomitant to pregnancy | 46% | 97% |
| Mean time since embryo transfer (days) | 10–11 | 40–42 |
| Thrombophyliaa | 19–26% | 41–48% |
| Most common location/ | Brain (stroke), extremities, heart (myocardial infarction) | Upper extremities, neck, intracranial |
| Characteristics . | ATE . | VTE . |
|---|---|---|
| Relative frequency (ATE versus VTE) | 1 | 2 |
| Concomitant to OHSS | 90–95% | 70–78% |
| Before final oocyte maturation trigger | 3% | 3% |
| Concomitant to pregnancy | 46% | 97% |
| Mean time since embryo transfer (days) | 10–11 | 40–42 |
| Thrombophyliaa | 19–26% | 41–48% |
| Most common location/ | Brain (stroke), extremities, heart (myocardial infarction) | Upper extremities, neck, intracranial |
Adapted from Stewart et al. (1997), Rao et al. (2005), Chan and Ginsberg (2006), Chan and Dixon (2008), Chan (2009) and Nelson (2009).
ATE, arterial thrombotic events; VTE, venous thromboembolism; OHSS, ovarian hyperstimulation.
aIncludes women heterozygous for methylene-tetra-hydro-folate-reductase (MTHFR).
A possible causal relationship between ovarian stimulation and thrombosis is supported by several biological investigations reporting on haemostatic parameters that have consistently shown pro-coagulant changes. During ovarian stimulation, there is indeed an increase in pro-coagulant factors such as von Willebrand factor, fibrinogen, factors V and VIII, a decrease of anticoagulant proteins such as antithrombin, proteins C and S, a development of resistance to activated protein C and an impaired fibrinolysis due to a decrease of tissue plasminogen activator and plasminogen activator inhibitor-1 (Chan and Dixon, 2008; Nelson, 2009; Westerlund et al., 2012). However, the clinical relevance of these modifications is not clear because the magnitude of these modifications is modest and the investigated parameters mostly remain within the normal ranges.
In the last few years, some observational studies have been published, shedding more light on the causal relationship between ovarian stimulation and thrombosis (Jacobsen et al., 2008a, b; Hansen et al., 2012, 2014; Rova et al., 2012; Henriksson et al., 2013). The main characteristics of these contributions are presented in Table III. The populations investigated largely overlap in the two Norwegian studies (Jacobsen et al., 2008a, b) and in the two Swedish studies (Rova et al., 2012; Henriksson et al., 2013). The studies have focused on VTE more than ATE, and have mostly included pregnant women. Only one paper report 20 cases (of VTE or ATE) in non-pregnant women in a 10-year interval. Results of these six studies are synthetically presented in Table IV. The main findings are the following: (i) the incidence of thrombosis following ovarian stimulation is generally very low; (ii) VTE essentially occurs in women achieving pregnancy; (iii) the risk of VTE peaks in pregnant women admitted to hospital for OHSS treatment; and (iv) the risk of VTE or ATE is not increased in women who do not become pregnant after ovarian stimulation, although this is based on a single study (Hansen et al., 2012).
Characteristics of the observational controlled studies on the relationship between ovarian stimulation and thrombosis.
| Authors, year . | Country . | Study period . | Study design . | Outcome . | Data collection . | Number of casesa . | Pregnancy state . |
|---|---|---|---|---|---|---|---|
| Jacobsen et al. (2008a) | Norway | 1990–2003 | Retrospective multicentre case–control (hospital controls) | VTE | Patients' charts + national register | 615 | Pregnancy and puerperium |
| Jacobsen et al. (2008b) | Norway | 1990–2003 | Retrospective multicentre case–control (population controls) | VTE | Patients' charts | 559 | Pregnancy and puerperium |
| Rova et al. (2012) | Sweden | 1998–2008 | Retrospective national cohort | VTE | National registries | 63 | Pregnancy and puerperium |
| Hansen et al. (2012) | Denmark | 1994–2005 | Retrospective national cohort | VTE and ATE | National registries | 20 (14 + 6) | Non-pregnant women |
| Henriksson et al. (2013) | Sweden | 1990–2008 | Retrospective national cohort | VTE and PE | National registries | 118 (99 + 19) | Pregnancy and puerperium |
| Hansen et al. (2014) | Denmark | 1995–2005 | Retrospective national cohort | VTE | National registries | 36 (26 + 10) | Pregnancy and puerperium |
| Authors, year . | Country . | Study period . | Study design . | Outcome . | Data collection . | Number of casesa . | Pregnancy state . |
|---|---|---|---|---|---|---|---|
| Jacobsen et al. (2008a) | Norway | 1990–2003 | Retrospective multicentre case–control (hospital controls) | VTE | Patients' charts + national register | 615 | Pregnancy and puerperium |
| Jacobsen et al. (2008b) | Norway | 1990–2003 | Retrospective multicentre case–control (population controls) | VTE | Patients' charts | 559 | Pregnancy and puerperium |
| Rova et al. (2012) | Sweden | 1998–2008 | Retrospective national cohort | VTE | National registries | 63 | Pregnancy and puerperium |
| Hansen et al. (2012) | Denmark | 1994–2005 | Retrospective national cohort | VTE and ATE | National registries | 20 (14 + 6) | Non-pregnant women |
| Henriksson et al. (2013) | Sweden | 1990–2008 | Retrospective national cohort | VTE and PE | National registries | 118 (99 + 19) | Pregnancy and puerperium |
| Hansen et al. (2014) | Denmark | 1995–2005 | Retrospective national cohort | VTE | National registries | 36 (26 + 10) | Pregnancy and puerperium |
All studies reported on in vitro fertilization.
VTE, venous thromboembolism; PE, pulmonary embolism; ATE, arterial thrombotic events.
aData in parentheses refer to the sum of singleton + twins.
Characteristics of the observational controlled studies on the relationship between ovarian stimulation and thrombosis.
| Authors, year . | Country . | Study period . | Study design . | Outcome . | Data collection . | Number of casesa . | Pregnancy state . |
|---|---|---|---|---|---|---|---|
| Jacobsen et al. (2008a) | Norway | 1990–2003 | Retrospective multicentre case–control (hospital controls) | VTE | Patients' charts + national register | 615 | Pregnancy and puerperium |
| Jacobsen et al. (2008b) | Norway | 1990–2003 | Retrospective multicentre case–control (population controls) | VTE | Patients' charts | 559 | Pregnancy and puerperium |
| Rova et al. (2012) | Sweden | 1998–2008 | Retrospective national cohort | VTE | National registries | 63 | Pregnancy and puerperium |
| Hansen et al. (2012) | Denmark | 1994–2005 | Retrospective national cohort | VTE and ATE | National registries | 20 (14 + 6) | Non-pregnant women |
| Henriksson et al. (2013) | Sweden | 1990–2008 | Retrospective national cohort | VTE and PE | National registries | 118 (99 + 19) | Pregnancy and puerperium |
| Hansen et al. (2014) | Denmark | 1995–2005 | Retrospective national cohort | VTE | National registries | 36 (26 + 10) | Pregnancy and puerperium |
| Authors, year . | Country . | Study period . | Study design . | Outcome . | Data collection . | Number of casesa . | Pregnancy state . |
|---|---|---|---|---|---|---|---|
| Jacobsen et al. (2008a) | Norway | 1990–2003 | Retrospective multicentre case–control (hospital controls) | VTE | Patients' charts + national register | 615 | Pregnancy and puerperium |
| Jacobsen et al. (2008b) | Norway | 1990–2003 | Retrospective multicentre case–control (population controls) | VTE | Patients' charts | 559 | Pregnancy and puerperium |
| Rova et al. (2012) | Sweden | 1998–2008 | Retrospective national cohort | VTE | National registries | 63 | Pregnancy and puerperium |
| Hansen et al. (2012) | Denmark | 1994–2005 | Retrospective national cohort | VTE and ATE | National registries | 20 (14 + 6) | Non-pregnant women |
| Henriksson et al. (2013) | Sweden | 1990–2008 | Retrospective national cohort | VTE and PE | National registries | 118 (99 + 19) | Pregnancy and puerperium |
| Hansen et al. (2014) | Denmark | 1995–2005 | Retrospective national cohort | VTE | National registries | 36 (26 + 10) | Pregnancy and puerperium |
All studies reported on in vitro fertilization.
VTE, venous thromboembolism; PE, pulmonary embolism; ATE, arterial thrombotic events.
aData in parentheses refer to the sum of singleton + twins.
Main findings of the observational controlled studies on the relationship between ovarian stimulation and thrombosis (see also Table III).
| Authors, year . | Outcome . | Subgroup . | Incidence in treated women . | OR, HR or IRR (95% CI) . |
|---|---|---|---|---|
| Jacobsen et al. (2008a) | VTE | Pregnancy | n.a. | OR = 4.4 (2.6–7.5)* |
| VTE | Puerperium | n.a. | OR = 2.2 (1.1–4.3)* | |
| Jacobsen et al. (2008b) | VTE | Pregnancy | n.a. | OR = 4.3 (2.0–9.4)* |
| VTE | Puerperium | n.a. | OR = 2.6 (0.8–8.5) | |
| Rova et al. (2012) | VTE | Pregnancy (all trimesters) | 2.7‰ | OR = 2.7 (2.1–3.6)* |
| VTE | First trimester | 1.7‰ | OR = 9.8 (6.7–14.3)* | |
| VTE | Second trimester | 0.3‰ | OR = 1.5 (0.6–3.6) | |
| VTE | Third trimester | 0.7‰ | OR = 1.1 (0.7–2.0) | |
| VTE | Puerperium | 0.6‰ | OR = 1.2 (0.6–2.0) | |
| VTE | non-OHSS | 0.8‰ | OR = 4.8 (2.7–8.7)* | |
| VTE | OHSS | 16.8‰ | OR = 99.7 (61.8–161.1)* | |
| VTE | Frozen embryos | 0.3‰ | OR = 1.7 (0.2–11.8) | |
| Hansen et al. (2012) | VTE | Non-pregnant at 6 months | 0.3‰ | IRR = 1.0 (0.4–2.0) |
| VTE | Non-pregnant at 12 months | 0.4‰ | IRR = 1.3 (0.7–2.1) | |
| ATE | Non-pregnant at 6 months | 0.1‰ | IRR = 0.4 (0.04–1.3) | |
| ATE | Non-pregnant at 12 months | 0.2‰ | IRR = 0.7 (0.3–1.6) | |
| Henriksson et al. (2013) | VTE | Pregnancy (all trimesters) | 4.2‰ | HR = 1.8 (1.4–2.2)* |
| VTE | First trimester | 1.5‰ | HR = 4.6 (3.0–7.2)* | |
| VTE | Second trimester | 1.0‰ | HR = 1.0 (0.5–2.0) | |
| VTE | Third trimester | 1.4‰ | HR = 1.0 (0.6–1.7) | |
| VTE | Puerperium | 1.0‰ | HR = 1.3 (0.8–2.0) | |
| PE | Pregnancy (all trimesters) | 0.8‰ | HR = 1.4 (0.9–2.4) | |
| PE | First trimester | 0.3‰ | HR = 7.0 (2.2–22.0)* | |
| PE | Second trimester | 0.2‰ | HR = 0.4 (0.1–3.2) | |
| PE | Third trimester | 0.3‰ | HR = 0.4 (0.1–1.7) | |
| PE | Puerperium | 0.1‰ | HR = 0.6 (0.2–2.0) | |
| Hansen et al. (2014) | VTE | Pregnancy (all trimesters) | 2.9‰ | IRR = 3.0 (2.1–4.3)* |
| VTE | First trimestera | n.r. | IRR = 5.9 (2.7–13.0)* | |
| VTE | Second trimestera | n.r. | IRR = 2.4 (0.9–6.6) | |
| VTE | Third trimestera | n.r. | IRR = 2.3 (1.4–3.8)* | |
| VTE | Puerperium | 2.8‰ | IRR = 1.7 (0.9–3.0) |
| Authors, year . | Outcome . | Subgroup . | Incidence in treated women . | OR, HR or IRR (95% CI) . |
|---|---|---|---|---|
| Jacobsen et al. (2008a) | VTE | Pregnancy | n.a. | OR = 4.4 (2.6–7.5)* |
| VTE | Puerperium | n.a. | OR = 2.2 (1.1–4.3)* | |
| Jacobsen et al. (2008b) | VTE | Pregnancy | n.a. | OR = 4.3 (2.0–9.4)* |
| VTE | Puerperium | n.a. | OR = 2.6 (0.8–8.5) | |
| Rova et al. (2012) | VTE | Pregnancy (all trimesters) | 2.7‰ | OR = 2.7 (2.1–3.6)* |
| VTE | First trimester | 1.7‰ | OR = 9.8 (6.7–14.3)* | |
| VTE | Second trimester | 0.3‰ | OR = 1.5 (0.6–3.6) | |
| VTE | Third trimester | 0.7‰ | OR = 1.1 (0.7–2.0) | |
| VTE | Puerperium | 0.6‰ | OR = 1.2 (0.6–2.0) | |
| VTE | non-OHSS | 0.8‰ | OR = 4.8 (2.7–8.7)* | |
| VTE | OHSS | 16.8‰ | OR = 99.7 (61.8–161.1)* | |
| VTE | Frozen embryos | 0.3‰ | OR = 1.7 (0.2–11.8) | |
| Hansen et al. (2012) | VTE | Non-pregnant at 6 months | 0.3‰ | IRR = 1.0 (0.4–2.0) |
| VTE | Non-pregnant at 12 months | 0.4‰ | IRR = 1.3 (0.7–2.1) | |
| ATE | Non-pregnant at 6 months | 0.1‰ | IRR = 0.4 (0.04–1.3) | |
| ATE | Non-pregnant at 12 months | 0.2‰ | IRR = 0.7 (0.3–1.6) | |
| Henriksson et al. (2013) | VTE | Pregnancy (all trimesters) | 4.2‰ | HR = 1.8 (1.4–2.2)* |
| VTE | First trimester | 1.5‰ | HR = 4.6 (3.0–7.2)* | |
| VTE | Second trimester | 1.0‰ | HR = 1.0 (0.5–2.0) | |
| VTE | Third trimester | 1.4‰ | HR = 1.0 (0.6–1.7) | |
| VTE | Puerperium | 1.0‰ | HR = 1.3 (0.8–2.0) | |
| PE | Pregnancy (all trimesters) | 0.8‰ | HR = 1.4 (0.9–2.4) | |
| PE | First trimester | 0.3‰ | HR = 7.0 (2.2–22.0)* | |
| PE | Second trimester | 0.2‰ | HR = 0.4 (0.1–3.2) | |
| PE | Third trimester | 0.3‰ | HR = 0.4 (0.1–1.7) | |
| PE | Puerperium | 0.1‰ | HR = 0.6 (0.2–2.0) | |
| Hansen et al. (2014) | VTE | Pregnancy (all trimesters) | 2.9‰ | IRR = 3.0 (2.1–4.3)* |
| VTE | First trimestera | n.r. | IRR = 5.9 (2.7–13.0)* | |
| VTE | Second trimestera | n.r. | IRR = 2.4 (0.9–6.6) | |
| VTE | Third trimestera | n.r. | IRR = 2.3 (1.4–3.8)* | |
| VTE | Puerperium | 2.8‰ | IRR = 1.7 (0.9–3.0) |
Data in parentheses are 95% CI. Only adjusted measurements of associations are reported.
VTE, venous thromboembolism; PE, pulmonary embolism; ATE, arterial thrombotic events; OR, odds ratio; HR, hazard ratio; IRR, incidence rate ratio.
aOnly singleton pregnancies included in the subgroup analyses.
*Statistically significant.
Main findings of the observational controlled studies on the relationship between ovarian stimulation and thrombosis (see also Table III).
| Authors, year . | Outcome . | Subgroup . | Incidence in treated women . | OR, HR or IRR (95% CI) . |
|---|---|---|---|---|
| Jacobsen et al. (2008a) | VTE | Pregnancy | n.a. | OR = 4.4 (2.6–7.5)* |
| VTE | Puerperium | n.a. | OR = 2.2 (1.1–4.3)* | |
| Jacobsen et al. (2008b) | VTE | Pregnancy | n.a. | OR = 4.3 (2.0–9.4)* |
| VTE | Puerperium | n.a. | OR = 2.6 (0.8–8.5) | |
| Rova et al. (2012) | VTE | Pregnancy (all trimesters) | 2.7‰ | OR = 2.7 (2.1–3.6)* |
| VTE | First trimester | 1.7‰ | OR = 9.8 (6.7–14.3)* | |
| VTE | Second trimester | 0.3‰ | OR = 1.5 (0.6–3.6) | |
| VTE | Third trimester | 0.7‰ | OR = 1.1 (0.7–2.0) | |
| VTE | Puerperium | 0.6‰ | OR = 1.2 (0.6–2.0) | |
| VTE | non-OHSS | 0.8‰ | OR = 4.8 (2.7–8.7)* | |
| VTE | OHSS | 16.8‰ | OR = 99.7 (61.8–161.1)* | |
| VTE | Frozen embryos | 0.3‰ | OR = 1.7 (0.2–11.8) | |
| Hansen et al. (2012) | VTE | Non-pregnant at 6 months | 0.3‰ | IRR = 1.0 (0.4–2.0) |
| VTE | Non-pregnant at 12 months | 0.4‰ | IRR = 1.3 (0.7–2.1) | |
| ATE | Non-pregnant at 6 months | 0.1‰ | IRR = 0.4 (0.04–1.3) | |
| ATE | Non-pregnant at 12 months | 0.2‰ | IRR = 0.7 (0.3–1.6) | |
| Henriksson et al. (2013) | VTE | Pregnancy (all trimesters) | 4.2‰ | HR = 1.8 (1.4–2.2)* |
| VTE | First trimester | 1.5‰ | HR = 4.6 (3.0–7.2)* | |
| VTE | Second trimester | 1.0‰ | HR = 1.0 (0.5–2.0) | |
| VTE | Third trimester | 1.4‰ | HR = 1.0 (0.6–1.7) | |
| VTE | Puerperium | 1.0‰ | HR = 1.3 (0.8–2.0) | |
| PE | Pregnancy (all trimesters) | 0.8‰ | HR = 1.4 (0.9–2.4) | |
| PE | First trimester | 0.3‰ | HR = 7.0 (2.2–22.0)* | |
| PE | Second trimester | 0.2‰ | HR = 0.4 (0.1–3.2) | |
| PE | Third trimester | 0.3‰ | HR = 0.4 (0.1–1.7) | |
| PE | Puerperium | 0.1‰ | HR = 0.6 (0.2–2.0) | |
| Hansen et al. (2014) | VTE | Pregnancy (all trimesters) | 2.9‰ | IRR = 3.0 (2.1–4.3)* |
| VTE | First trimestera | n.r. | IRR = 5.9 (2.7–13.0)* | |
| VTE | Second trimestera | n.r. | IRR = 2.4 (0.9–6.6) | |
| VTE | Third trimestera | n.r. | IRR = 2.3 (1.4–3.8)* | |
| VTE | Puerperium | 2.8‰ | IRR = 1.7 (0.9–3.0) |
| Authors, year . | Outcome . | Subgroup . | Incidence in treated women . | OR, HR or IRR (95% CI) . |
|---|---|---|---|---|
| Jacobsen et al. (2008a) | VTE | Pregnancy | n.a. | OR = 4.4 (2.6–7.5)* |
| VTE | Puerperium | n.a. | OR = 2.2 (1.1–4.3)* | |
| Jacobsen et al. (2008b) | VTE | Pregnancy | n.a. | OR = 4.3 (2.0–9.4)* |
| VTE | Puerperium | n.a. | OR = 2.6 (0.8–8.5) | |
| Rova et al. (2012) | VTE | Pregnancy (all trimesters) | 2.7‰ | OR = 2.7 (2.1–3.6)* |
| VTE | First trimester | 1.7‰ | OR = 9.8 (6.7–14.3)* | |
| VTE | Second trimester | 0.3‰ | OR = 1.5 (0.6–3.6) | |
| VTE | Third trimester | 0.7‰ | OR = 1.1 (0.7–2.0) | |
| VTE | Puerperium | 0.6‰ | OR = 1.2 (0.6–2.0) | |
| VTE | non-OHSS | 0.8‰ | OR = 4.8 (2.7–8.7)* | |
| VTE | OHSS | 16.8‰ | OR = 99.7 (61.8–161.1)* | |
| VTE | Frozen embryos | 0.3‰ | OR = 1.7 (0.2–11.8) | |
| Hansen et al. (2012) | VTE | Non-pregnant at 6 months | 0.3‰ | IRR = 1.0 (0.4–2.0) |
| VTE | Non-pregnant at 12 months | 0.4‰ | IRR = 1.3 (0.7–2.1) | |
| ATE | Non-pregnant at 6 months | 0.1‰ | IRR = 0.4 (0.04–1.3) | |
| ATE | Non-pregnant at 12 months | 0.2‰ | IRR = 0.7 (0.3–1.6) | |
| Henriksson et al. (2013) | VTE | Pregnancy (all trimesters) | 4.2‰ | HR = 1.8 (1.4–2.2)* |
| VTE | First trimester | 1.5‰ | HR = 4.6 (3.0–7.2)* | |
| VTE | Second trimester | 1.0‰ | HR = 1.0 (0.5–2.0) | |
| VTE | Third trimester | 1.4‰ | HR = 1.0 (0.6–1.7) | |
| VTE | Puerperium | 1.0‰ | HR = 1.3 (0.8–2.0) | |
| PE | Pregnancy (all trimesters) | 0.8‰ | HR = 1.4 (0.9–2.4) | |
| PE | First trimester | 0.3‰ | HR = 7.0 (2.2–22.0)* | |
| PE | Second trimester | 0.2‰ | HR = 0.4 (0.1–3.2) | |
| PE | Third trimester | 0.3‰ | HR = 0.4 (0.1–1.7) | |
| PE | Puerperium | 0.1‰ | HR = 0.6 (0.2–2.0) | |
| Hansen et al. (2014) | VTE | Pregnancy (all trimesters) | 2.9‰ | IRR = 3.0 (2.1–4.3)* |
| VTE | First trimestera | n.r. | IRR = 5.9 (2.7–13.0)* | |
| VTE | Second trimestera | n.r. | IRR = 2.4 (0.9–6.6) | |
| VTE | Third trimestera | n.r. | IRR = 2.3 (1.4–3.8)* | |
| VTE | Puerperium | 2.8‰ | IRR = 1.7 (0.9–3.0) |
Data in parentheses are 95% CI. Only adjusted measurements of associations are reported.
VTE, venous thromboembolism; PE, pulmonary embolism; ATE, arterial thrombotic events; OR, odds ratio; HR, hazard ratio; IRR, incidence rate ratio.
aOnly singleton pregnancies included in the subgroup analyses.
*Statistically significant.
Finally, it has to be pointed out that there is no observational study specifically reporting on the risk of VTE and ATE in women undergoing ovarian stimulation for fertility preservation. However, given that women undergoing fertility preservation are not going to undergo embryo transfer (so no pregnancy will be observed in short term) and OHSS is unlikely to develop (see below), the risks of VTE and ATE is presumably extremely low, if any. Of note, we failed to identify any case report documenting a thrombotic event in women undergoing ovarian stimulation for fertility preservation.
Ovarian hyperstimulation syndrome
Ovarian hyperstimulation syndrome (OHSS) is an uncommon but frightening complication of ovarian stimulation. The syndrome is mainly characterized by increased vascular permeability and a leakage to the extravascular space, a phenomenon mostly consequent to the peripheral rise in vascular endothelial growth factor (VEGF) (Soares et al., 2008; Gómez et al., 2010). The fluid shift can cause tension ascites that, in most severe cases, can also be transmitted into the thoracic cavity leading to pleural effusions (Grossman et al., 2010; Nastri et al., 2010). The pathogenesis of the syndrome is still unclear but a pivotal role of human chorionic gonadotrophin (hCG) is unquestionable. Indeed, there are two forms of OHSS, early and late, reflecting the different exposure of women to hCG. The early form, which develops soon after oocyte retrieval and rapidly regresses on its own, is related to the pharmacological administration of hCG, that is indeed commonly given to induce final oocyte maturation 34–36 h prior to oocyte retrieval. The symptoms of this early form of OHSS regress concomitantly with the clearance of hCG from blood. Conversely, the late form lasts several weeks, affects women achieving pregnancy, is more severe and begins 2–3 weeks after oocyte retrieval, i.e. when hCG secreted by the trophoblast becomes detectable at the peripheral level. The increased severity and duration of the late form reflects the persistent and rapidly increasing hCG serum level that typically occurs at the beginning of pregnancy (Budev et al., 2005).
The precise pathogenic mechanisms causing the rise in thrombosis risk in women with OHSS are unknown but it is plausible that the elevated peripheral levels of estrogens, the hemoconcentration typically occurring in affected women and the loss of plasma protein may on the whole determine a condition favouring thrombosis. Interestingly, the two possible explanations for the localization of VTE to the upper part of the body both rely on the leakage into the extravascular space, which characterizes OHSS. Bauersachs et al. hypothesized that this particular localization may be consequent to the increased drainage of peritoneal fluid with inflammatory properties and high estrogen levels through the thoracic duct into the subclavian veins (Bauersachs et al., 2007). Salomon et al. suggested that these events may be consequent to mechanical obstruction from rudimentary brachial cysts which fill with fluid during OHSS (Salomon et al., 2009).
The pivotal role of hCG in the development of OHSS has opened new scenarios of utmost clinical relevance. It has been hypothesized and subsequently demonstrated that replacing hCG with gonadotrophin releasing hormone (GnRH) agonists to induce final oocyte maturation prior to oocyte collection markedly prevents the development of the syndrome without affecting the quality of the retrieved oocytes (Humaidan et al., 2011, 2013; Evers, 2013). Cases of OHSS when using this approach are rare (Fatemi et al., 2014). This option can be used only in ovarian stimulation cycles with GnRH antagonists and the unique limitation is the potentially detrimental effect on the luteal phase, an argument of no relevance within the context of fertility preservation procedures since an altered luteal phase will only affect the implantation of the embryo and not the quality of the oocytes retrieved. Not surprisingly, it has been recently claimed that, in women at risk of OHSS, segmentation of the IVF cycle using a GnRH agonist for final oocyte maturation and then freezing all oocytes or embryos with subsequent replacement of the embryos in the context of a frozen embryo transfer abolishes the risk of OHSS and therefore prevents thrombosis (Nelson, 2013).
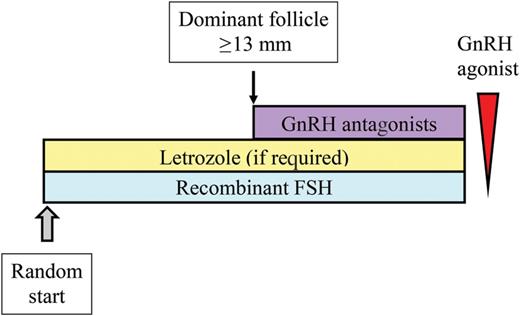
Suggested protocol of ovarian stimulation for fertility preservation. Women should initiate ovarian stimulation at the time of referral at the infertility unit regardless of the phase of the cycle to avoid delays in the initiation of chemotherapy (‘random start’) (Cakmak and Rosen 2013; Cakmak et al., 2013). In the luteal phase and in the early follicular phase, gonadotrophins can be administered alone until a dominant follicle with a mean diameter ≥13 mm is visualized at transvaginal sonographic monitoring. At this time, GnRH antagonists should be initiated to prevent spontaneous ovulation. If women are referred in the late follicular phase, gonadotrophins and GnRH antagonists should be initiated concomitantly or, alternatively, hCG can be given to induce ovulation and gonadotrophin stimulation can be started 2 days later. Concomitant administration of Letrozole 5 mg daily is indicated in women with hormonally sensitive cancers (Reddy and Oktay, 2012). Final oocyte maturation prior to oocyte retrieval should be triggered by the administration of GnRH agonist (0.2 mg). This is equally effective as hCG but prevents OHSS (Humaidan et al., 2011; Garcia-Velasco, 2012). The dose of gonadotrophins to be administered should be tailored to the characteristics of the women and in particular to their age and antral follicular count (AFC) since these two variables can be easily collected at the time of referral (hormonal tests need at least some days to be obtained). In most cases, a dose of 150–225 IU daily is adequate.
Discussion
Unfortunately, because specific data on the risk of thrombosis in women undergoing ovarian stimulation for fertility preservation are lacking, a firm recommendation for the prevention of VTE and ATE cannot be drawn and inferences from the available evidences obtained in women without malignancies should be made with caution. Nonetheless, even if specific observational studies are needed to definitely disentangle this issue, we believe that some clinical indications can be given. In this regard, the following main points are highlighted.
The clinical profile of women with malignancies referring for fertility preservation is more favourable in terms of thrombotic risk than that of the general oncologic population. They are young, the disease is mostly at an early stage and the most common diagnosis, i.e. breast cancer, is at low risk (Table I).
In women without malignancies, VTE and ATE following ovarian stimulation is rare. Even if both OHSS and its thrombotic complications are presumed to be underreported across the world, the data obtained from the available population-based studies are actually reassuring. In the normal ART setting (i.e. women performing the procedure with transfer of fresh embryos), antithrombotic prophylaxis is indicated only in highly selected cases such as those achieving a pregnancy and developing OHSS or those with a personal history of thrombotic events.
The available evidence suggests that pregnancy is a necessary predisposing factor for VTE. Venous events following ovarian stimulation are indeed almost universally observed in pregnant women and develop when pregnancy is well established (40–42 days following embryo transfer according to case reports, 60–68 days according to a population-based study) (Rova et al., 2012). On this basis, it can be speculated that VTE is not a concern in women undergoing ovarian stimulation for fertility preservation.
Conversely, ATE may theoretically occur in women undergoing ovarian stimulation for fertility preservation. The unique available cohort study on women undergoing IVF but failing to conceive did not show an increased risk (Hansen et al., 2012) but there were available case reports (Table II). Of relevance here is that, in contrast to VTE, ATE tends to occur with a shorter delay (10–11 days following embryo transfer) and can occur also in women failing to become pregnant.
OHSS plays a crucial role in determining ovarian stimulation-related thrombosis, and in particular ATE. However, the late and more severe form of OHSS invariably develops if pregnancy is achieved and cannot occur in women undergoing ovarian stimulation for fertility preservation. Thus, the risk is limited to the early form of OHSS.
The early form of OHSS can nowadays be effectively prevented using specific regimen of ovarian stimulation (Fig. 1).
Further evidence based on large case series are obviously needed to draw robust conclusions. The main theoretical and unsolved question here is the interactive effects of malignancy and ovarian stimulation in terms of risk of thrombosis. Up to now, we have assumed that the ovarian stimulation and malignant-related risks are independent but we cannot exclude that they may boost each other. In other words, we have convincing evidence showing that the risks of thrombosis in women undergoing ovarian stimulation and oocyte retrieval without embryo transfer is very low but we do not have sufficient data to infer that this may be also true in women with malignancies. Of potential relevance here is that the pro-coagulant modifications reported during ovarian stimulation may persist for several weeks and they may thus actually overlap with the initiation of chemotherapy, a well-known condition exposing to an increased risk of thrombosis. Similarly, the surgically related increased risk of thrombosis may also last several weeks, thus potentially overlapping with the effects of ovarian stimulation that is typically performed shortly after surgery. If the detrimental effects of ovarian stimulation is exclusively additive, we do not estimate that this point should be given major relevance within the balance of factors and the decision to prescribe antithrombotic prophylaxis should only be made on the basis of other factors such as personal and family history of thrombosis, thrombophilia abnormalities (if known), type of malignancy, chemotherapeutic regimens, hospitalization and blood tests results. This recommendation may be reconsidered if data showing a multiplicative rather than an additive effect emerge. Up to now however, there is no evidence to support this theoretical apprehension.
In conclusion, based on the available evidence, a strategy of systematic antithrombotic prophylaxis during fertility preservation for cancer patients is not justified as it exposes women to side effects and discomfort and may negatively affect compliance. Women should therefore be informed about the risk of thrombosis but they should also be concomitantly reassured regarding the magnitude of this risk. Antithrombotic prophylaxis should be administered only to particular subgroups of women. They include those developing early OHSS (and low-dose aspirin would be the preferred option) and those who may benefit from antithrombotic prophylaxis regardless of ovarian stimulation, i.e. those with the most thrombogenic malignancies, those undergoing cancer treatments requiring hospitalization (such as surgery or high doses chemotherapies) and those at high individual risk (mainly those with a personal history of thrombosis). In general, a comprehensive evaluation and a tailored decision should be taken but should give modest relevance to ovarian stimulation (with the exception for those developing OHSS). Finally, there is also no indication for testing thrombophilia since the available evidence showed that it may play only a secondary role in the context of ovarian stimulation-related thrombosis.
Authors' roles
E.S. conceived and designed the study and drafted the manuscript. F.A.P. and F.R. retrieved and interpreted the data on cancer and thrombosis and revised the manuscript. F.F. and F.M. retrieved and interpreted the data on ovarian stimulation and thrombosis and revised the manuscript. I.M. conceived and designed the study and revised the manuscript.
Funding
No funding was requested for this study.
Conflict of interest
None declared.