-
PDF
- Split View
-
Views
-
Cite
Cite
Sharon Tsay, Alexander Kallen, Brendan R Jackson, Tom M Chiller, Snigdha Vallabhaneni, Approach to the Investigation and Management of Patients With Candida auris, an Emerging Multidrug-Resistant Yeast, Clinical Infectious Diseases, Volume 66, Issue 2, 15 January 2018, Pages 306–311, https://doi.org/10.1093/cid/cix744
Close - Share Icon Share
Abstract
Candida auris is an emerging, multidrug-resistant yeast that can spread in healthcare settings. It can cause invasive infections with high mortality and is difficult to identify using traditional yeast identification methods. Candida auris has been reported in more than a dozen countries, and as of August 2017, 112 clinical cases have been reported in the United States. Candida auris can colonize skin and persist in the healthcare environment, allowing for transmission between patients. Prompt investigation and aggressive interventions, including notification to public health agencies, implementation of contact precautions, thorough environmental cleaning and disinfection, infection control assessments, contact tracing and screening of contacts to assess for colonization, and retrospective review of microbiology records and prospective surveillance for cases at laboratories are all needed to limit the spread of C. auris. This review summarizes the current recommended approach to manage cases and control transmission of C. auris in healthcare facilities.
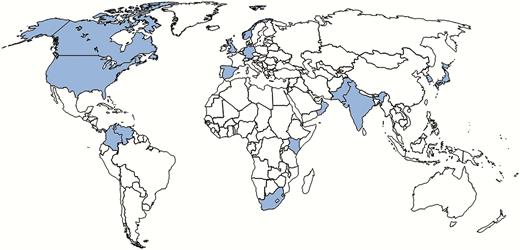
Candida auris is an emerging, multidrug-resistant yeast that can cause invasive infections and has been associated with outbreaks in healthcare settings (Box 1). Candida auris was first described in 2009 after isolation from external ear discharge from a patient in Japan [1]. Reports of bloodstream infections, in which persistent infection despite treatment and drug resistance to fluconazole and amphotericin B were described, followed quickly thereafter from South Korea and India [2–7]. Subsequently, C. auris infections have been reported in more than a dozen countries [8–13] (Figure 1). Although attributable mortality is unknown, 30%–60% of patients with C. auris infection have died [8]. In some places, C. auris now accounts for an increasing proportion of candidemia cases; an unknown pathogen before 2009, C. auris caused 4%–8% of candidemia in Indian intensive care units during 2011–2012 and 38% of candidemia in 1 Kenyan hospital during 2010–2013 [11, 14]. Whole-genome sequencing of C. auris isolates has revealed 4 distinct clades that cluster geographically (South Asia, East Asia, South Africa, and South America) with a high degree of relatedness within clades, suggesting independent emergence with transmission within a geographic area rather than a single emergence and spread [8].
Countries from which Candida auris has been reported, as of July 2017. Canada, Germany, Japan, Norway, and Kuwait have each reported a single case of C. auris. Larger numbers of cases have been reported in Colombia, India, Israel, Kenya, Oman, Pakistan, Panama, South Korea, Spain, South Africa, the United Kingdom, and Venezuela. Current case counts of C. auris for all countries are not available. United States case counts are available on the Centers for Disease Control and Prevention website. Most US cases are concentrated in the New York City and New Jersey area, though at least 7 other states have reported cases as of August 2017.
It can cause invasive infections with high mortality.
59% all-cause mortality in early studies
Majority of cases in the United States to date have been bloodstream infections (candidemia)
It is difficult to identify.
Most often misidentified as Candida haemulonii by conventional biochemical methods
MALDI-TOF or DNA sequencing are required to identify C. auris
It is often multidrug resistant.
Most isolates are resistant to fluconazole
Some are resistant to amphotericin B
Small proportion are resistant to echinocandins
Resistance to all 3 classes of antifungals has been observed in other world regions
It can spread in healthcare settings.
Persists on patients’ skin and the healthcare environment, allowing for transmission to occur between patients in healthcare facilities
Outbreaks of C. auris have been reported in several countries
In April 2015, a specialty hospital in the United Kingdom identified a C. auris outbreak among patients in a cardiothoracic intensive care unit [15]. Testing revealed colonization of additional patients and C. auris on hospital surfaces and equipment. Control of the outbreak required implementation of aggressive infection control practices, including use of contact precautions and thrice-daily room disinfection with bleach. Although outbreaks of Candida parapsilosis have been reported, Candida infections are usually thought to result from autoinfection with host flora rather than transmission from external sources [16–18]. The UK outbreak clearly demonstrated that C. auris can be transmitted in healthcare settings [15].
In response to global reports and the UK hospital outbreak, the Centers for Disease Control and Prevention (CDC) issued a clinical alert to US healthcare facilities about C. auris in June 2016 [19]. As of August 21, 2017, 232 (112 from clinical cultures, 120 screened contacts) were reported to have C. auris infection or colonization [20]. All but 1 of these cases occurred in 2015 or later, suggesting that this organism has emerged only recently in the United States. Nearly all cases have occurred within limited geographic areas. Given the recent emergence and geographic concentration of cases, an opportunity exists to control the spread of this organism before it becomes more widespread.
Experience with other multidrug-resistant organisms (MDROs) suggests that an early, aggressive approach to control the organism when newly emerging is more effective and efficient in controlling transmission than responding when the organism is more widespread [21, 22]. This review summarizes the current recommended approach to manage cases and control transmission of C. auris in healthcare facilities. This effort requires coordination between all involved stakeholders, including healthcare facilities, clinicians, public health practitioners, and industry. Many of the principles for containment of C. auris are similar to those for other MDROs.
CANDIDA AURIS IDENTIFICATION
The first step in controlling C. auris is identification. Candida auris can be misidentified when using traditional biochemical methods [23]. Depending on the identification method used (eg, VITEK-2, API-20C, BD-Phoenix, Microscan), C. auris should be suspected when an isolate is identified as a certain Candida species, such as Candida haemulonii, Candida famata, Candida sake, Candida catenulata, or Rhodotorula glutinis, or if species identification cannot be obtained [23]. Currently, accurate identification for C. auris can be performed by Vitek MS and Bruker Biotyper brand matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) using research use–only databases. Molecular methods based on sequencing of the D1–D2 region of the 28s rDNA or internal transcribed spacer region can also reliably identify C. auris [12, 24–26]. Clinicians should be aware of the diagnostic instruments used in their hospital laboratories and their ability to detect C. auris [27]. Clinical laboratories can request testing of suspect C. auris isolates from their state or regional public health laboratory or the CDC. Laboratories should also consider reviewing historical microbiology records for suspect isolates (eg, C. haemulonii) to identify missed cases of C. auris.
ANTIFUNGAL RESISTANCE
Antifungal susceptibility testing for all clinically relevant Candida isolates is recommended in the 2016 Infectious Diseases Society of America (IDSA) Clinical Practice Guidelines for Candidiasis [18]. Resistance to ≥1 antifungal drugs in an isolate with ambiguous identification should raise the suspicion of C. auris and prompt further testing. In 1 collection of 54 C. auris isolates from 5 countries, 93% were resistant to fluconazole, 35% to amphotericin B, and 7% to echinocandins. In total, 41% were resistant to ≥2 antifungal classes [8]. In the United States, 86% of the first 35 cases were resistant to fluconazole, 43% to amphotericin B, and 3% (n = 1) to echinocandins [20]. Although minimum inhibitory concentration breakpoints have not been established for C. auris, breakpoints are suggested based on those used for closely related Candida species and expert opinion, especially for amphotericin B, for which no breakpoints exist for any Candida species [28]. Tentative minimum inhibitory concentration breakpoints for resistance include ≥32 µg/mL for fluconazole, ≥2 µg/mL for amphotericin B, ≥2 µg/mL for caspofungin, and ≥4 µg/mL for anidulafungin and micafungin.
TREATMENT OF CANDIDA AURIS INFECTION
Consultation with an infectious disease specialist is highly recommended. Despite its multidrug-resistant nature, most C. auris isolates to date have been susceptible to echinocandins. The recommended initial therapy for clinically relevant infections with C. auris in adults is an echinocandin at standard dosing. Patients should be monitored closely for resolution of infection given that resistance to echinocandins has been documented and because resistance has emerged on serial isolates from a single patient after exposure to the drug. Switching to or adding liposomal amphotericin B (5 mg/kg daily) could be considered if the patient is clinically unresponsive to echinocandin treatment or has fungemia for >5 days. Other management considerations for C. auris are similar to Candida infections with other species; practitioners should refer to the 2016 IDSA Clinical Practice Guidelines [18].
CONTROLLING CANDIDA AURIS TRANSMISSION IN HEALTHCARE SETTINGS
The presence of a single case in a healthcare facility should prompt an aggressive response and investigation because C. auris can cause healthcare-associated outbreaks. Patients can remain colonized on their skin and other body sites indefinitely after resolution of invasive infections, allowing C. auris to be shed into the healthcare environment, where it persists on surfaces and can be transmitted to other patients [15, 29]. Containment efforts should focus on identifying patients who are infected or colonized with C. auris and implementing infection control interventions, including hand hygiene, contact precautions, and thorough environmental cleaning and disinfection [28].
RESPONSE TO A CASE OF CANDIDA AURIS
As soon as C. auris is suspected, the patient should be placed in a single room under contact precautions until definitive identification is available. When C. auris is confirmed at a healthcare facility, the following actions should be taken: notify state or local health departments and the CDC; institute infection control measures; perform a detailed case review; identify colonized patients through contact review; and review microbiology records (Box 2).
Notify public health agency of confirmed or suspected C. auris cases
Report to the Centers for Disease Control and Prevention at candidaauris@cdc.gov
Place patient in a single room if possible and institute standard and contact precautions
Reinforce and enhance hand hygiene practices
Institute thorough environmental cleaning and disinfection of the patient care area
Use an Environmental Protection Agency–registered disinfectant active against Clostridium difficile for routine and terminal disinfection
Implement contact tracing and testing to identify other patients colonized with C. auris
Composite swab of axilla and groin to assess for skin colonization
Swab roommates and those with longest overlapping contact with the case patient
Conduct microbiology records review
Review past microbiology records (at least for the preceding 1 year) for suspect or confirmed cases of C. auris at the institution.
Set up enhanced surveillance for C. auris in the laboratory serving the healthcare facility to detect any future cases of C. auris immediately
Notify
A case of C. auris should be reported as soon as possible to the state or local health department and the CDC. The CDC has established an email address for reporting: candidaauris@cdc.gov.
Institute Infection Control Measures
Standard and contact precautions with placement of the patient in a single room is recommended. Adherence to proper hand hygiene with alcohol-based hand rub or soap and water should be reinforced [30].
Candida auris can persist on surfaces in the healthcare settings [31]. Thorough daily and terminal cleaning of the patient’s room and any mobile equipment used should be performed with an Environmental Protection Agency–registered hospital-grade disinfectant effective against Clostridium difficile spores [32]. Preliminary laboratory testing suggests that certain commonly used hospital disinfectants, notably quaternary ammonia compounds, are not sufficiently effective against C. auris.
Perform Detailed Case Review
Basic information about the case patient, including demographic characteristics and clinical history, should be obtained. In the United States, patients with C. auris were found to have had on average 3 healthcare facility encounters in the 90 days preceding their diagnosis; the majority had been admitted to a high-acuity long-term care facility (LTCF). It is important to obtain records of recent healthcare encounters, including stays at other acute care hospitals and LTCFs, to assess for possible transmission at the other facilities.
Taking a detailed travel history, especially receipt of healthcare in countries where C. auris cases have been reported, is important (Figure 1). Several US case patients have had a recent history of hospitalization in countries with a large burden of C. auris, including India, Pakistan, South Africa, and Venezuela. Based on whole-genome sequencing at the CDC, most isolates from US patients are closely related to isolates from South Asia and South America.
Identify Colonized Patients Through Contact Investigation
Contact investigation should be conducted to identify persons who were exposed to an incident case to detect transmission. As part of a detailed C. auris case review, it is important to identify epidemiologically linked patients for possible screening because colonized patients pose a risk for transmission. Current or past roommates are considered at high risk for becoming colonized and should be screened even if they are no longer admitted to the facility. Other potential contacts might include patients who overlapped on a ward with a patient with C. auris and patients who moved into a room recently vacated by a patient with C. auris, especially if cleaning practices were suboptimal.
To identify colonized people, ≥1 high-yield body sites should be sampled with a swab. For example, studies evaluating screening for methicillin-resistant Staphylococcus aureus (MRSA) have shown nares to be the highest yield site, ranging 71%–84% [33–35]. Yield can be increased further if additional body sites are included; for example, MRSA detection is >90% if nares, throat, and perineum are all sampled [35]. Because no studies on sampling sites exist for C. auris, early cases were sampled from multiple body sites (including nares, ears, oropharynx, axilla, groin, and rectum) to determine those with highest yield. Approximately 90% of cases were positive by axilla or groin swab. Nares was the second most commonly positive body site. Screening of epidemiologically linked patients with a composite swab of the bilateral axillae and groin is recommended; additional body sites, including nares, may be sampled if feasible. Unlike screening for MRSA, laboratory processing of swabs taken to identify C. auris colonization is not currently commercially available and should be coordinated through local or state health departments and the CDC. All patients identified as colonized with C. auris should be managed in the same manner as the index patient and placed in a single room on contact precautions. It is also important to ensure that the patient’s status and required infection control measures are communicated at the time of transfer to another healthcare facility.
No known decolonization methods have been established. Candida auris is susceptible to chlorhexidine in vitro and has been used in certain settings for source control; however, despite daily chlorhexidine bathing, patients described in the United Kingdom continued to be colonized with C. auris [15]. Further study is needed on efficacy of chlorhexidine and other products for decolonization before recommendations for their use can be made.
Review of Microbiology Records
Because C. auris is commonly misidentified as other Candida species, clinical laboratories serving the affected facility should review microbiology records to identify other suspected cases, as should clinical laboratories serving other facilities where the patient recently received care. These reviews should include specimens from all body sites and include ≥1 year of microbiology records, preferably as far back as 2015. Laboratories that have identified a case of C. auris should be on heightened alert for additional cases of C. auris and should consider performing species identification on all Candida isolates identified from any body site from patients on an affected unit for a limited time until there is no evidence of ongoing transmission.
RESPONSE TO MORE THAN ONE CASE OF CANDIDA AURIS
Although a case of C. auris is enough to prompt an investigation, >1 case raises the concern for transmission. When >1 patient with C. auris is identified at a healthcare facility, including patients identified through screening, the following additional actions are recommended: perform infection control assessments; perform additional case finding; consider environmental or healthcare worker sampling in limited settings; consider regional notification to laboratories and other healthcare facilities.
Perform Infection Control Assessments
Infection control assessments should be conducted to look for opportunities for improvement. These assessments offer an opportunity to collaborate with staff and provide comprehensive education that benefits the facility beyond the control of C. auris. Particular areas to target during these assessments include hand hygiene, contact precautions, and environmental cleaning and disinfection.
Hand hygiene assessment should include evaluating the availability of appropriate resources, like alcohol-based hand rub and ready access to sinks with soap and water. Use monitoring programs to ensure staff adherence and to target ongoing education and encouragement. When evaluating the implementation of contact precautions, assess the availability of personal protective equipment, clear signage outside patient rooms, and staff adherence. In resource-limited settings, facilities may have to consider cohorting patients with C. auris together; however, if patients have multiple MDROs, care should be taken not to cohort patients with different MDROs together.
In the UK outbreak, thorough cleaning and disinfection with sodium hypochlorite–based products and hydrogen peroxide vapor was reported to be a key factor in eventual control of the outbreak [15]. Environmental cleaning and disinfection in healthcare facilities should be a collaborative effort between environmental services, patient support staff, and healthcare workers. Training on use of the proper agent, mixed to the proper concentration (if required), and appropriate contact time are essential to ensuring surfaces are adequately disinfected. Samples from C. auris patient rooms in the United States after terminal cleaning with sodium hypochlorite–based products have not yielded growth of C. auris. If these measures fail to stop transmission, closure of an affected ward for a certain period may be needed to interrupt transmission [15].
Perform Additional Case Finding
Broader patient screening should be strongly considered in facilities with >1 patient with C. auris, especially in high-acuity nursing homes, where substantial transmission of C. auris has occurred. Point-prevalence surveys (PPSs) for C. auris colonization of affected units or an entire facility can rapidly assess the extent of transmission and identify patients who may be sources of ongoing transmission.
Results from the initial PPS can help determine the need for further screening. For example, if multiple patients on a particular ward are colonized, the next step might be to screen the entire floor or facility. In general, if further transmission is detected on the PPS, additional PPSs are warranted after interventions are undertaken to assess the impact of these interventions on transmission.
Consider Environmental or Healthcare Worker Sampling in Limited Settings
Early US investigations of C. auris included environmental sampling of surfaces in case patients’ hospital rooms during active infection, and many different types of surfaces yielded positive cultures for C. auris. Based on these results, contamination of affected patients’ rooms is expected, and environmental sampling is generally not recommended. However, environmental sampling could be considered if epidemiologic evidence links specific environmental sources to C. auris transmission or in situations where ongoing transmission is identified despite adherence to recommended interventions.
Whereas transient contamination of hands of healthcare personnel (HCP) is likely to play a role in C. auris transmission, the role of chronic HCP colonization is unclear. Systematic sampling of the hands, nose, axilla, groin, and throat of 258 HCP was conducted as part of the UK investigation and identified a single HCP with a positive nares swab who later tested negative from the same site, suggesting transient carriage [15]. Screening of HCP should be considered only if an epidemiologic investigation suggests HCPs as a likely source or in situations where ongoing transmission is identified despite adherence to recommended interventions.
Consider Regional Notification to Laboratories and Other Healthcare Facilities
When ≥1 C. auris case is identified, local or state health departments may consider notifying laboratories and healthcare facilities in the region to raise awareness and aid in additional case finding. Laboratory messaging from public health agencies should include information about when to suspect and how to identify C. auris and highlight the importance of determining Candida species and antifungal susceptibility testing [18]. Microbiology record reviews can also be requested on a wider scale at other facilities in the region to identify other suspect cases. These laboratories should also be encouraged to conduct prospective surveillance for new cases. Because other Candida species do not typically cause outbreaks, heightened infection control practices are not typically recommended in the control of Candida infections. Therefore, it is particularly important to educate HCPs in the region about the distinct ability of C. auris to spread in healthcare settings and about current control recommendations to improve identification, notification, and implementation of infection control measures.
UNANSWERED QUESTIONS AND ONGOING WORK
Even as more becomes known about C. auris, many unanswered questions remain that directly affect the implications of testing and identifying cases. These questions include the following:
Where did C. auris come from and why is it emerging now?
What should salvage treatment consist of in cases where the organism is resistant to the 3 main classes of antifungals?
How can C. auris colonization be rapidly detected?
How long can a person remain colonized with C. auris?
What methods are effective for reducing the burden of C. auris colonization?
What are risk factors for infection in a patient colonized with C. auris?
How effective are the recommended infection control strategies at containing C. auris?
What is the prevalence of C. auris in the community and does transmission occur there?
How rapidly and under what circumstances does C. auris become resistant to antifungal drugs?
Future studies will aim to answer these questions in addition to others to better understand C. auris and how best to contain its spread.
CONCLUSION
Candida auris is a newly emerging, often multidrug-resistant fungal pathogen, similar in many ways to bacterial MDROs with which hospital epidemiologists and clinicians are already familiar. The ability of C. auris to colonize the skin, persist in the healthcare environment, and cause healthcare-associated outbreaks has changed the way we think about Candida infections. Prevention and containment of C. auris requires many of the same interventions that are used to contain other MDROs that spread in healthcare settings, and it is critical that these interventions are implemented early and thoroughly.
Notes
Acknowledgments. The authors thank their local and state health partners and laboratories for their ongoing work in investigating C. auris cases. The authors also thank Anastasia P. Litvintseva, Shawn R. Lockhart, Rory M. Welsh, Nancy A. Chow, Lalitha Gade, Elizabeth L. Berkow, and Meghan Bentz in the Centers for Disease Control and Prevention (CDC) Mycotic Diseases Branch laboratory for support in processing samples, confirming isolates, and performing antifungal susceptibility testing and whole genome sequencing.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.





Comments