-
PDF
- Split View
-
Views
-
Cite
Cite
George Petrikkos, Anna Skiada, Olivier Lortholary, Emmanuel Roilides, Thomas J. Walsh, Dimitrios P. Kontoyiannis, Epidemiology and Clinical Manifestations of Mucormycosis, Clinical Infectious Diseases, Volume 54, Issue suppl_1, February 2012, Pages S23–S34, https://doi.org/10.1093/cid/cir866
Close - Share Icon Share
Abstract
Mucormycosis is an emerging angioinvasive infection caused by the ubiquitous filamentous fungi of the Mucorales order of the class of Zygomycetes. Mucormycosis has emerged as the third most common invasive mycosis in order of importance after candidiasis and aspergillosis in patients with hematological and allogeneic stem cell transplantation. Mucormycosis also remains a threat in patients with diabetes mellitus in the Western world. Furthermore, this disease is increasingly recognized in recently developed countries, such as India, mainly in patients with uncontrolled diabetes or trauma. Epidemiological data on this type of mycosis are scant. Therefore, our ability to determine the burden of disease is limited. Based on anatomic localization, mucormycosis can be classified as one of 6 forms: (1) rhinocerebral, (2) pulmonary, (3) cutaneous, (4) gastrointestinal, (5) disseminated, and (6) uncommon presentations. The underlying conditions can influence clinical presentation and outcome. This review describes the emerging epidemiology and the clinical manifestations of mucormycosis.
During the past 2 decades, mucormycosis has emerged as an important fungal infection with high associated mortality rates. Zygomycoses are uncommon, frequently fatal diseases caused by fungi of the class Zygomycetes (consisting of the orders Mucorales and Entomophthorales) with distinct patterns of clinical infection. The majority of human cases are caused by Mucorales fungi; therefore, the terms mucormycosis and zygomycosis are used interchangeably (the term phycomycosis is also used) [1, 2]. The Mucorales species most often recovered from clinical specimens are those of the genera Rhizopus (the most common genus associated with mucormycosis), Lichtheimia (formerly known as Absidia and Mycocladus), and Mucor. Species of other Zygomycetes genera, such as Rhizomucor, Saksenaea, Cunninghamella, and Apophysomyces, are less common [2–7]. Fungi of the order Entomophthorales are uncommon pathogens, with infections typically restricted to tropical areas, and produce chronic cutaneous and subcutaneous infections. In general, these infections occur in immunocompetent hosts and progress locally via direct extension into adjacent tissues but rarely are angioinvasive or become disseminated [2, 8–10]. In contrast, Mucorales species are vasotropic, causing tissue infarctions, and the mucormycosis spectrum ranges from cutaneous, rhinocerebral, and sinopulmonary to disseminated and frequently fatal infections, especially in immunocompromised hosts [11, 12]. The importance of Mucorales species has grown in recent years as the number of patients with predisposing factors for mucormycosis has increased dramatically.
EPIDEMIOLOGY
Most human infections result from inhalation of fungal sporangiospores that have been released in the air or direct inoculation of organisms into disrupted skin or mucosa [13]. The Mucorales are ubiquitous in nature, but their precise ecology remains to be determined; they are thermotolerant and are usually found in decaying organic matter. Cases with mucormycosis have been reported from all over the world. Seasonal variation in Mucorales infection is possible. In a study from Israel, 16 of 19 reported cases of rhino-orbitocerebral mucormycosis (ROCM) occurred in autumn [14]. In another study from Japan, a similar seasonal variation among hematology patients was noted, with 6 of 7 cases of pulmonary mucormycosis having developed from August to September [15].
Differences in the epidemiology of mucormycosis seem to exist between developed and developing countries. In developed countries, the disease remains uncommon and, at present, is mostly seen in patients with diabetes mellitus and hematological malignancies (HMs) undergoing chemotherapy and those who have received allogeneic stem cell transplants [1]. In contrast, in developing countries, especially in India, mucormycosis cases, although sporadic, occur mainly in patients with uncontrolled diabetes or trauma [2, 16].
There are several factors that limit our ability to accurately determine the exact incidence of mucormycosis. Autopsy rates, the “gold standard” approach, have been in continuous decline globally during the last decades. Nevertheless, mucormycosis remains an uncommon disease, even in high-risk patients, and represents 8.3%–13% of all fungal infections encountered at autopsy in such patients [17–19]. Postmortem prevalence evaluation shows that mucormycosis is 10–50-fold less frequent than candidiasis or aspergillosis with a frequency of 1–5 cases per 10 000 autopsies [17–21].
The challenges to establish a clinical diagnosis of mucormycosis add to the difficulty of obtaining a clear view of the epidemiology of this disease. Patients are often treated presumptively for mucormycosis, but cases may be missed due to lack of histological and microbiological proof [22]. The incorporation of modern, minimally invasive techniques for safer tissue sampling and the use of molecular techniques for the identification of the pathogen show promise for clarification of many ambiguous clinical scenarios in the near future [23]. Laboratory diagnosis is discussed in greater depth elsewhere in this supplement.
The literature contains few population-based studies [24–31]. These studies differ in capture periods, populations, and definition or case ascertainment procedures (Table 1 and Figure 1). Nearly 2 decades ago, in the era before highly active antiretroviral therapy, an active population-based surveillance study in San Francisco, California, from 1992 to 1993 revealed that the annual incidence of mucormycosis was 1.7 cases per 1 million individuals (∼500 cases per year) [24]. A more recent study in a more general population in Spain found a lower incidence (0.43 cases/1 million inhabitants, or 0.62/100 000 hospital admissions) [25]. The analysis of hospital records in France showed an increasing incidence from 0.7/million in 1997 to 1.2/million in 2006 (P < .001) [26]. In the largest review of mucormycosis cases in the English-language literature, Roden et al compiled 929 cases of mucormycosis from 1885 to 2004 and showed that an increasing proportion of immunocompromised patients with mucormycosis was reported in the 1980s and 1990s [27]. Patients with HMs or who underwent hematopoietic stem cell transplantation (HSCT) represented 22% of the cases (17% and 5%, respectively). In the largest registry of the European Confederation of Medical Mycology Working Group, 230 cases occurring between 2005 and 2007 were analyzed and found to occur in patients with hematological malignancies (44%), trauma (15%), HSCT (9%), and diabetes mellitus (9%) [28]. During the same period, an exhaustive analysis was performed at the country level in all hospitals of France and revealed a total of 101 cases of mucormycoses (60 proven/41 probable), mostly in men (58%) >50 years old (mean age, 50.7 ± 19.9 years). Fifty percent of the cases were in patients with hematological malignancies with or without stem cell transplantation, 23% in patients with diabetes, and 18% in patients with trauma. Notably, HIV infection does not inherently predispose to mucormycosis unless associated with illicit intravenous drug use, neutropenia, diabetes mellitus, or corticosteroids.
Underlying Conditions in Patients With Mucormycosis as Presented in Various Studies
| Characteristics of Studies | Underlying Conditions, % of Cases | |||||||||||
| Reference | Countries of Origin for Cases | Prospective Studya | Multicenter Study | Time Period | Cases, No. | HM | DM | SOM/SOT | DFO | HIV | AI/CO | Trauma/No Underlying Disease |
| Roden et alb [27] | Global | NA | NA | 1885–2004 | 929 | 21.0 | 36.0 | 7.0 | 6.0 | 2.0 | 1.0 | 19.0 |
| Bitar et alc [26] | France | No | Yes | 1997–2006 | 53 | 17.3 | 16.2 | 7.1 | … | 4.9 | … | 54.4 |
| Pagano et al [34] | Italy | Yes | Yes | 2004–2007 | 60 | 61.7 | 18.0 | 1.7 | … | 1.7 | 3.3 | 40.0 |
| Saegeman et al [185] | Belgium | No | Yes | 2000–2009 | 31 | 77.0 | 6.4 | 13.0 | … | 3.0 | … | 13.0 |
| Rüping et al [33] | Global | Yes | Yes | 2006–2009 | 41 | 63.4 | 17.1 | 9.8 | … | … | … | … |
| Skiada et al [28] | Europe | Yes | Yes | 2005–2007 | 230c | 55.0 | 17.0 | 9.0 | 1.0 | 2.0 | 7.0 | 20.0 |
| Chakrabarti et al [16] | India | Yes | No | 2006–2007 | 178 | 1.1 | 73.6 | 0.6 | … | … | … | 19.1 |
| Characteristics of Studies | Underlying Conditions, % of Cases | |||||||||||
| Reference | Countries of Origin for Cases | Prospective Studya | Multicenter Study | Time Period | Cases, No. | HM | DM | SOM/SOT | DFO | HIV | AI/CO | Trauma/No Underlying Disease |
| Roden et alb [27] | Global | NA | NA | 1885–2004 | 929 | 21.0 | 36.0 | 7.0 | 6.0 | 2.0 | 1.0 | 19.0 |
| Bitar et alc [26] | France | No | Yes | 1997–2006 | 53 | 17.3 | 16.2 | 7.1 | … | 4.9 | … | 54.4 |
| Pagano et al [34] | Italy | Yes | Yes | 2004–2007 | 60 | 61.7 | 18.0 | 1.7 | … | 1.7 | 3.3 | 40.0 |
| Saegeman et al [185] | Belgium | No | Yes | 2000–2009 | 31 | 77.0 | 6.4 | 13.0 | … | 3.0 | … | 13.0 |
| Rüping et al [33] | Global | Yes | Yes | 2006–2009 | 41 | 63.4 | 17.1 | 9.8 | … | … | … | … |
| Skiada et al [28] | Europe | Yes | Yes | 2005–2007 | 230c | 55.0 | 17.0 | 9.0 | 1.0 | 2.0 | 7.0 | 20.0 |
| Chakrabarti et al [16] | India | Yes | No | 2006–2007 | 178 | 1.1 | 73.6 | 0.6 | … | … | … | 19.1 |
Abbreviations: AI/CO, autoimmune diseases and/or corticosteroid therapy; DFO, deferroxamine therapy; DM, diabetes mellitus; HIV, human immunodeficiency virus; HM, hematological malignancies and/or hematopoietic stem cell transplantation; NA, not applicable; SOM/SOT, solid organ malignancy and/or solid organ transplantation.
Depicted studies are from registries collecting passive surveillance data; no study involved active surveillance of cases.
This was a review of reported cases in the literature.
In this study, 19 patients (8%) had >1 underlying disease, so the total is >100%.
Underlying Conditions in Patients With Mucormycosis as Presented in Various Studies
| Characteristics of Studies | Underlying Conditions, % of Cases | |||||||||||
| Reference | Countries of Origin for Cases | Prospective Studya | Multicenter Study | Time Period | Cases, No. | HM | DM | SOM/SOT | DFO | HIV | AI/CO | Trauma/No Underlying Disease |
| Roden et alb [27] | Global | NA | NA | 1885–2004 | 929 | 21.0 | 36.0 | 7.0 | 6.0 | 2.0 | 1.0 | 19.0 |
| Bitar et alc [26] | France | No | Yes | 1997–2006 | 53 | 17.3 | 16.2 | 7.1 | … | 4.9 | … | 54.4 |
| Pagano et al [34] | Italy | Yes | Yes | 2004–2007 | 60 | 61.7 | 18.0 | 1.7 | … | 1.7 | 3.3 | 40.0 |
| Saegeman et al [185] | Belgium | No | Yes | 2000–2009 | 31 | 77.0 | 6.4 | 13.0 | … | 3.0 | … | 13.0 |
| Rüping et al [33] | Global | Yes | Yes | 2006–2009 | 41 | 63.4 | 17.1 | 9.8 | … | … | … | … |
| Skiada et al [28] | Europe | Yes | Yes | 2005–2007 | 230c | 55.0 | 17.0 | 9.0 | 1.0 | 2.0 | 7.0 | 20.0 |
| Chakrabarti et al [16] | India | Yes | No | 2006–2007 | 178 | 1.1 | 73.6 | 0.6 | … | … | … | 19.1 |
| Characteristics of Studies | Underlying Conditions, % of Cases | |||||||||||
| Reference | Countries of Origin for Cases | Prospective Studya | Multicenter Study | Time Period | Cases, No. | HM | DM | SOM/SOT | DFO | HIV | AI/CO | Trauma/No Underlying Disease |
| Roden et alb [27] | Global | NA | NA | 1885–2004 | 929 | 21.0 | 36.0 | 7.0 | 6.0 | 2.0 | 1.0 | 19.0 |
| Bitar et alc [26] | France | No | Yes | 1997–2006 | 53 | 17.3 | 16.2 | 7.1 | … | 4.9 | … | 54.4 |
| Pagano et al [34] | Italy | Yes | Yes | 2004–2007 | 60 | 61.7 | 18.0 | 1.7 | … | 1.7 | 3.3 | 40.0 |
| Saegeman et al [185] | Belgium | No | Yes | 2000–2009 | 31 | 77.0 | 6.4 | 13.0 | … | 3.0 | … | 13.0 |
| Rüping et al [33] | Global | Yes | Yes | 2006–2009 | 41 | 63.4 | 17.1 | 9.8 | … | … | … | … |
| Skiada et al [28] | Europe | Yes | Yes | 2005–2007 | 230c | 55.0 | 17.0 | 9.0 | 1.0 | 2.0 | 7.0 | 20.0 |
| Chakrabarti et al [16] | India | Yes | No | 2006–2007 | 178 | 1.1 | 73.6 | 0.6 | … | … | … | 19.1 |
Abbreviations: AI/CO, autoimmune diseases and/or corticosteroid therapy; DFO, deferroxamine therapy; DM, diabetes mellitus; HIV, human immunodeficiency virus; HM, hematological malignancies and/or hematopoietic stem cell transplantation; NA, not applicable; SOM/SOT, solid organ malignancy and/or solid organ transplantation.
Depicted studies are from registries collecting passive surveillance data; no study involved active surveillance of cases.
This was a review of reported cases in the literature.
In this study, 19 patients (8%) had >1 underlying disease, so the total is >100%.
Predisposing Conditions
The most important conditions predisposing to mucormycosis, according to various studies, include malignant hematological disease with or without stem cell transplantation, prolonged and severe neutropenia, poorly controlled diabetes mellitus with or without diabetic ketoacidosis, iron overload, major trauma, prolonged use of corticosteroids, illicit intravenous drug use, neonatal prematurity and malnourishment [1, 3, 27]. Antifungal agents with no activity against Zygomycetes, such as voriconazole and caspofungin, have also been implicated in breakthrough zygomycosis [27, 28]. Apart from these host-related risk factors, a number of cases have been—at least partially—attributed to the hospital environment. Nosocomial mucormycosis has been associated with exposure to heavy air fungal loads because of construction work, contaminated air filters, or a variety of healthcare-associated procedures and devices, such as contaminated wound dressings, transdermal nitrate patches, intravenous catheters, tongue depressors, and even allopurinol pills [30]. Iatrogenic mini-outbreaks also have occurred [31, 32]. An exhaustive literature review on the topic is included in the present supplement.
HM and Hematopoietic Stem Cell Transplantation
Among patients with HM, those with acute myelogenous leukemia (AML) are at the highest risk for mucormycosis, with incidences ranging from 1% to 8% [15, 33–39]. Mucormycosis is less common in other acute or chronic HMs [39]. In patients with either autologous or allogeneic stem cell transplantation, the frequency of mucormycosis is lower than that observed in patients with AML, ranging from 0.9% to 2.0%, with the highest incidence observed in patients with graft-versus-host disease [40–46]. Some studies have suggested a rising incidence of mucormycosis in HM units. At the University of Texas MD Anderson Cancer Center, the number of cases increased from 8/100 000 admissions in 1989–1993 to 17/100 000 admissions in 1994–1998. The overall incidence of mucormycosis among those with HMs at this center at autopsy was 1.9% for the period 1994–1998 versus only 0.7% during the period 1989–1998 [37]. A similar incidence of 2.1% at autopsy in patients with acute leukemia was reported in a study from Japan [15]. Further insight into the epidemiology of mucormycosis is anticipated with analysis of data from prospective networks, such as that from the Centers for Disease Control and Prevention TRANSNET (Parks and Kontoyiannis, submitted) and the Prospective Antifungal Therapy (PATH) Alliance. However, these networks focused only on transplant patients (TRANSNET) or immunosuppressed patients in general (PATH Alliance), in whom the incidence of mucormycosis may be higher [45]. Surveillance programs that reach beyond the setting of HSCT would further advance understanding of the epidemiology of this rare disease [45, 47].
Solid Organ Malignancies and Solid Organ Transplantation
Mucormycosis constitutes a small proportion of invasive fungal infections in solid organ transplant (SOT) recipients; however, the disease is also associated with a high mortality rate. The estimated incidence ranges from 0.4% to 16.0% depending on the SOT type [26, 27, 47–50]: the range is 0.2%– 1.2% in renal transplant recipients, 0%–1.6% in liver transplant recipients, 0%–0.6% in heart transplant recipients, and 0%–1.5% in lung transplant recipients [51–63]. In a retrospective study and literature review, neutropenia was absent in SOT recipients, as were acidosis with and without hyperglycemia and deferoxamine (DFO) use [50]. In contrast, all patients underwent chronic immunosuppression, typically with high doses of systemic corticosteroids. Furthermore, dissemination of mucormycosis to distant organs occurred often after rejection and its treatment. Dissemination occurred preferentially to skin and soft tissues but not the brain. In a prospective, matched, case-controlled study of mucormycosis in SOT recipients, renal failure, diabetes mellitus, and prior voriconazole and/or caspofungin use were associated with an increased risk of mucormycosis [52]. In contrast, tacrolimus use was associated with a decreased risk. Liver transplant recipients were more likely to have disseminated disease and had earlier development of mucormycosis after transplantation versus other SOT recipients (median, 0.8 vs 5.7 months).
Diabetes Mellitus and Ketoacidosis.
Authors have reported diabetes mellitus as a predisposing factor for mucormycosis in 36%–88% of cases [27, 64–73]. Patients with uncontrolled hyperglycemia, particularly those with ketoacidosis, are the most susceptible [11, 69, 70]. Mucormycosis may be the first manifestation in some patients with undiagnosed diabetes mellitus [72], but it is rarely observed in those with metabolically controlled diabetes [71]. Type 1, type 2, and secondary diabetes mellitus are all reportedly risk factors for mucormycosis [73].
The epidemiology of mucormycosis in diabetic patients is evolving. Roden et al [27] showed that diabetic patients represented 36% of 929 reported cases, but there was a decreased incidence of mucormycosis in diabetics over time. This contrasts with the increased prevalence of diabetes worldwide [74]. Researchers have speculated that treatment with statins, used widely for metabolic syndromes in the West, has a role in this decreased incidence, because statins are active against some Zygomycetes [74, 75]. However, mucormycosis remains a formidable threat in diabetics. A recent nationwide retrospective study in France showed a 9% annual increase in mucormycosis incidence in diabetics [26]. In another retrospective study from 2 American teaching and tertiary care hospitals, 83% of patients with ROCM were diabetics; 41% of them had no known history of diabetes [76]. Data from a study in a tertiary care center in India were also alarming, as 74% of patients with mucormycosis had uncontrolled diabetes; in 43% of these cases, diabetes was diagnosed for the first time [16].
These findings underline the association between poor diabetes control and socioeconomic status. Unless complications occur, low-income individuals avoid seeking medical attention, because healthcare is largely unaffordable to them. Furthermore, almost 80% of type 2 diabetes mellitus deaths occur in low- and middle-income individuals [77]. Many of these mucormycosis cases could have been prevented with diabetes control programs.
Corticosteroid Use and Rheumatic Diseases.
Chronic corticosteroid-based therapy is another primary risk factor that enhances a patient’s susceptibility to mucormycosis by causing defects in macrophages and neutrophils and/or steroid-induced diabetes [3]. In the few cases of mucormycosis in patients with systemic lupus erythematosus reported in the English-language literature [77–91], the infection apparently could present in any clinical form, but disseminated mucormycosis was common and the mortality rate was very high (88%) [78]. Additional predisposing factors for opportunistic mucormycosis include hypocomplementemia, nephrotic syndrome, uremia, leukopenia, and diabetes mellitus. Opportunistic mucormycosis occasionally occurs in patients with other autoimmune diseases. Importantly, in patients with Wegener granulomatosis, mucormycosis may mimic a relapse of the underlying disease and go undiagnosed [92].
Iron Overload and Chelation Therapy With DFO.
Therapy with DFO, an iron chelator used to treat iron and/or aluminum overload in dialysis recipients, reportedly is a risk factor for angioinvasive mucormycosis [93, 94]. A report of an international registry of mycoses showed that 78% of dialysis recipients with mucormycosis received DFO [95]. Besides DFO, iron overload, either transfusional or caused by dyserythropoiesis, is a risk factor for mucormycosis [42, 96, 97]. The most common presentation of mucormycosis in patients receiving DFO seems to be the disseminated form (44%), and this presentation is associated with high mortality rates, reaching 80% [25, 95]. Fortunately, DFO is no longer used because of the introduction of novel iron chelators such as deferasirox (Exjade) and deferiprone, which do not predispose patients to mucormycosis.
Prolonged Use of Voriconazole.
The introduction and widespread use of Aspergillus-active agents, especially voriconazole, mainly in patients with HMs and recipients of hematopoietic stem cell transplants at high risk for mucormycosis is linked with increased incidence of mucormycosis in several institutions worldwide [98–106]. Recent prospective randomized studies comparing prophylaxes using voriconazole with those using fluconazole or itraconazole in allogeneic transplant recipients did not confirm this finding, however [107, 108]. Both of these studies enrolled patients at low risk for invasive mold infections, including mucormycosis, so the controversy about the use of voriconazole remains. Yet, despite uncertainty about this risk, clinicians should be aware that mucormycosis can develop in high-risk patients receiving voriconazole, because double fungal infections are not uncommon.
HIV or AIDS
Mucormycosis in patients with human immunodeficiency virus (HIV) or AIDS is very rare. In a large retrospective study of 1630 autopsies of patients who died of AIDS from 1984 to 2002, Antinori et al [107] observed only 2 patients with mucormycosis. This relatively low frequency reflects the uncommon occurrence of mucormycosis in HIV-infected patients compared with other immunocompromised populations. [27, 28] Most mucormycosis cases in HIV-infected patients are associated with intravenous drug use [109–112].
No Underlying Disease.
A considerable proportion of patients with mucormycosis have no apparent immune deficiency [25]. These patients typically have primary cutaneous mucormycosis associated with trauma or burns. Authors have reported iatrogenic factors as causes of mucormycosis, including surgical trauma and use of contaminated bandages, adhesive dressings, wooden tongue depressors, and central venous catheters [32, 113].
Mucormycosis in Children.
Mucormycosis in children is very rare. Zaoutis et al [114] reviewed all available English-language reports of pediatric mucormycosis cases before 2004. They found a total of 157 case patients (64% male), with a median age of 5 years. Twenty-eight (18%) patients had HMs, and 9 (6%) had undergone HSCT. Authors reported an additional 30 pediatric cases from 2004 to 2008 [115].
CLINICAL MANIFESTATIONS
The clinical hallmark of invasive mucormycosis is tissue necrosis resulting from angioinvasion and subsequent thrombosis. In most cases, the infection is rapidly progressive and results in death unless underlying risk factors (ie, metabolic acidosis) are corrected and aggressive treatment with antifungal agents and surgical excision is instituted. Based on its clinical presentation and anatomic site, invasive mucormycosis is classified as one of the following 6 major clinical forms: (1) rhinocerebral, (2) pulmonary, (3) cutaneous, (4) gastrointestinal, (5) disseminated, and (6) uncommon rare forms, such as endocarditis, osteomyelitis, peritonitis, and renal infection [116–118]. Any of the species of the Mucorales may cause infection at these sites.
The most common reported sites of invasive mucormycosis have been the sinuses (39%), lungs (24%), and skin (19%) [25]. Dissemination developed in 23% of these cases. The overall mortality rate for the disease is 44% in diabetics, 35% in patients with no underlying conditions, and 66% in patients with malignancies. The mortality rate varied with the site of infection and host: 96% of patients with disseminated infections, 85% with gastrointestinal infections, and 76% with pulmonary infections died. In children, mucormycosis manifested as cutaneous, gastrointestinal, rhinocerebral, and pulmonary infections in 27%, 21%, 18%, and 16% of cases, respectively, in one study [114]. The skin and gut are affected more frequently in children than in adults.
Pulmonary Mucormycosis
Pulmonary mucormycosis occurs most often in neutropenic patients with cancer undergoing induction chemotherapy and those who have undergone HSCT and have graft-versus-host disease [119]. The overall mortality rate in patients with pulmonary mucormycosis is high (76%); it is even higher in severely immunosuppressed patients [25]. The clinical features of pulmonary mucormycosis are nonspecific and cannot be easily distinguished from those of pulmonary aspergillosis. Patients usually present with prolonged high-grade fever (>38°C) that is unresponsive to broad-spectrum antibiotics. Nonproductive cough is a common symptom, whereas hemoptysis, pleuritic chest pain, and dyspnea are less common. In rare circumstances, pulmonary mucormycosis can present as an endobronchial or tracheal lesion, especially in diabetics. Endobronchial mucormycosis can cause airway obstruction, resulting in lung collapse, and can lead to invasion of hilar blood vessels with subsequent massive hemoptysis [120–122]. Pulmonary mucormycosis may invade lung-adjacent organs, such as the mediastinum, pericardium, and chest wall [123].
The signs of pulmonary mucormycosis on chest images are also nonspecific and indistinguishable from those of pulmonary aspergillosis. The most frequent findings include infiltration, consolidation, nodules, cavitations, atelectasis, effusion, posterior tracheal band thickening, hilar or mediastinal lymphadenopathy, and even normal findings [124–126]. The air crescent sign may be observed and is similar to that seen with pulmonary aspergillosis [127, 128]. In a study of computed tomography (CT) scan features in 45 patients with HMs who had pulmonary mucormycosis or invasive pulmonary aspergillosis, Chamilos et al [129] found that the presence of multiple lung nodules (≥10) and pleural effusion on initial CT scans was an independent predictor of pulmonary mucormycosis. Researchers have observed that the CT finding of a reversed halo sign, a focal round area of ground-glass attenuation surrounded by a ring of consolidation, is more common in patients with mucormycosis than in those with other invasive pulmonary fungal infections [130].
Rhinocerebral Mucormycosis
Rhinocerebral mucormycosis (ROCM) is the most common form of mucormycosis in patients with diabetes mellitus [27, 28]. It may also occur in patients with underlying malignancies, recipients of hematopoietic stem cell or solid organ transplants, and individuals with other risk factors [39]. The infection develops after inhalation of fungal sporangiospores into the paranasal sinuses. The infection may then rapidly extend into adjacent tissues. Upon germination, the invading fungus may spread inferiorly to invade the palate, posteriorly to invade the sphenoid sinus, laterally into the cavernous sinus to involve the orbits, or cranially to invade the brain [131]. The fungus invades the cranium through either the orbital apex or cribriform plate of the ethmoid bone and ultimately kills the host. Occasionally, cerebral vascular invasion can lead to hematogenous dissemination of the infection with or without development of mycotic aneurysms [132]. The initial symptoms of ROCM are consistent with those of sinusitis and periorbital cellulitis and include eye and/or facial pain and facial numbness followed by blurry vision [3]. Signs and symptoms that suggest mucormycosis in susceptible individuals include multiple cranial nerve palsies, unilateral periorbital facial pain, orbital inflammation, eyelid edema, blepharoptosis, proptosis, acute ocular motility changes, internal or external ophthalmoplegia, headache, and acute vision loss.
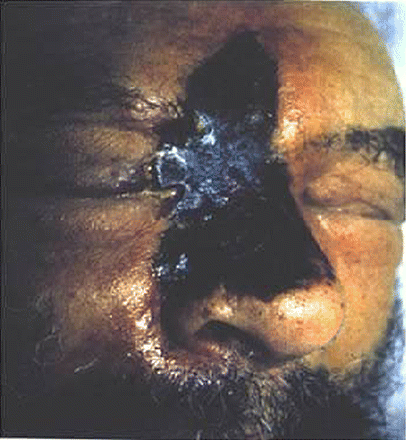
A black necrotic eschar is the hallmark of mucormycosis (Figure 2). However, the absence of this finding should not exclude the possibility of mucormycosis. Fever is variable and may be absent in up to half of cases. The white blood cell count is typically elevated as long as the patient has functioning bone marrow.
Necrotic facial eschar of a patient with rhinocerebral mucormycosis.
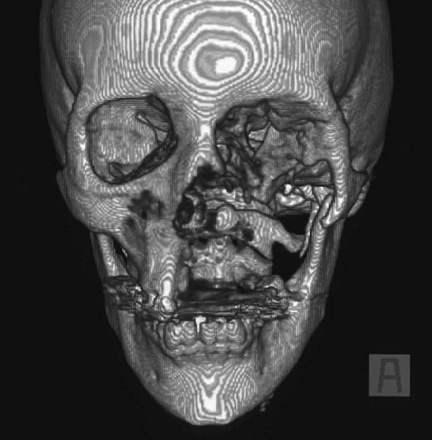
Preoperative contrast-enhanced CT is useful in defining the extent of ROCM. Such CT scans show the edematous mucosa, fluid filling the ethmoid sinuses, and destruction of periorbital tissues and bone margins [133]. Although sinus CT is the preferred imaging modality for evaluating signs of invasion (Figure 3), bone destruction is often seen only late in the infection course after soft-tissue necrosis has occurred.
Three-dimensional computed tomographic scan of the facial skeleton of a patient with rhinocerebral mucormycosis showing extensive skeletal defects after orbitomaxillary resection.
Magnetic resonance (MR) imaging is quite useful in identifying the intradural and intracranial extent of ROCM, cavernous sinus thrombosis, and thrombosis of cavernous portions of the internal carotid artery. Contrast-enhanced MR imaging can also demonstrate perineural spread of the infection. Although evidence of infection of orbital soft tissues may be seen on CT scans, MR imaging is more sensitive for this [114, 135]. Still, as with CT scans, patients with early ROCM may have normal MR images , and high-risk patients should always undergo surgical exploration with biopsy analysis of the suspected areas of infection. Nevertheless, imaging studies are nonspecific for ROCM, and diagnosing ROCM almost always requires histopathological evidence of fungal tissue invasion. Given the rapidly progressive nature of ROCM and marked increase in the mortality rate when the fungus penetrates the cranium, any diabetic patient with a headache and visual changes is a candidate for prompt evaluation using imaging studies and nasal endoscopy to rule out mucormycosis.
Cutaneous Mucormycosis
Cutaneous mucormycosis results from direct inoculation of fungal spores in the skin, which may lead to disseminated disease. The reverse (dissemination from internal organs to the skin) is very rare [1, 27]. Roden et al [27] noted such reverse dissemination in only 6 cases (3%). Depending on the extent of the infection, cutaneous mucormycosis is classified as localized when it affects only the skin or subcutaneous tissue; deep extension when it invades muscle, tendons, or bone; and disseminated when it involves other noncontiguous organs [136].
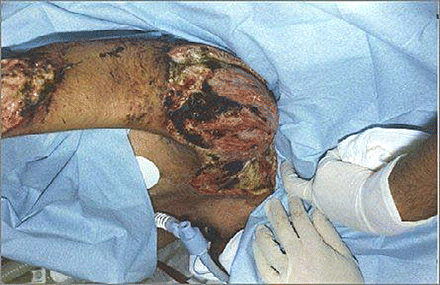
The clinical manifestations of cutaneous mucormycosis vary. Its onset may be gradual, and it may progress slowly, or it may be fulminant, leading to gangrene and hematogenous dissemination [137–139]. The typical presentation of cutaneous mucormycosis is a necrotic eschar accompanied by surrounding erythema and induration. However, a nonspecific erythematous macule, although small and apparently insignificant, may be the cutaneous manifestation of disseminated disease in an immunosuppressed patient [138]. Authors have reported other, less common presentations of cutaneous mucormycosis, such as superficial lesions with only slightly elevated circinate and squamous borders resembling tinea corporis [136], targetoid plaques with outer erythematous rims and ecchymotic or blackened necrotic centers [139], and, in patients with open wounds, lesions with a cottonlike appearance resembling that of bread mold [140, 141]. When cutaneous mucormycosis presents with necrotic eschars, these lesions may mimic pyoderma gangrenosum, bacterial synergistic gangrene, or other infections produced by bacteria or fungi (Figure 4) [142].
Cutaneous necrotizing mucormycosis of the right upper limb of a burn victim.
Gastrointestinal Mucormycosis
Gastrointestinal mucormycosis is uncommon and seldom diagnosed in living patients. In such cases, diagnosis is delayed, and the mortality rate is as high as 85% [27]. Only 25% of gastrointestinal mucormycosis cases are diagnosed antemortem, and authors have reported the disease mainly in premature neonates, malnourished children, and individuals with HMs, diabetes mellitus, or a history of corticosteroid use [143–148]. Gastrointestinal mucormycosis is acquired by ingestion of pathogens in foods such as fermented milk and dried bread products [13]. Consumption of fermented porridges and alcoholic drinks derived from corn may promote gastric mucormycosis. Use of spore-contaminated herbal and homeopathic remedies is also linked with gastrointestinal mucormycosis [148, 149]. Finally, one group of authors reported on a series of cases of gastrointestinal mucormycosis presumably transmitted orally with sporangiospore contaminated tongue depressors used for oropharyngeal examinations in a hematology-oncology clinic [144].
Gastrointestinal mucormycosis can occur in any part of the alimentary system, but the stomach is most commonly affected, followed by the colon and ileum [147, 151, 152]. The infection usually presents with an appendiceal, cecal, or ileac mass or gastric perforation that may be associated with frequently massive upper gastrointestinal tract bleeding [145, 148, 151–156]. In premature neonates, gastrointestinal mucormycosis presents as necrotizing enterocolitis, whereas in neutropenic patients, it does so as a masslike appendiceal or ileal lesion [3, 157]. Neutropenic fever, typhlitis, and hematochezia also can occur in neutropenic patients. Diagnosis of gastrointestinal mucormycosis is usually delayed, because its nonspecific presentation requires a high degree of suspicion, leading to early use of endoscopic biopsy analysis. Gastrointestinal mucormycosis can also involve the liver, spleen, and pancreas. The fungus can invade bowel walls and blood vessels, resulting in bowel perforation, peritonitis, sepsis, and massive gastrointestinal hemorrhage, which is the most common cause of death [153, 157].
Disseminated Mucormycosis
Mucormycosis in one organ can spread hematogenously to other organs [84, 158–162]. The organ most commonly associated with dissemination is the lung. Dissemination also occurs from the alimentary tract, burns, and extensive cutaneous lesions. Although the brain is a common site of spread, metastatic lesions may also be found in the liver, spleen, heart, and other organs [163]. Patients with iron overload (especially those receiving deferoxamine), profound immunosuppression (eg, recipients of allogeneic stem cell transplants having graft-versus-host disease treated with corticosteroids), or profound neutropenia and active leukemia are the classic groups at risk for disseminated mucormycosis [2, 11, 95, 163]. The symptoms and evolution of disseminated mucormycosis vary widely, reflecting the host as well as the location and degree of vascular invasion and tissue infarction in the affected organs. The variable presentation of disseminated mucormycosis requires a high index of suspicion to enable early diagnosis. Postmortem diagnosis of disseminated mucormycosis is quite common. A metastatic skin lesion is an important hallmark in early diagnosis. Without appropriate treatment, disseminated mucormycosis is always fatal [161].
Uncommon Forms of Mucormycosis
Other less common or unusual focal forms of mucormycosis include endocarditis, osteomyelitis, peritonitis, and pyelonephritis. Specifically, mucormycosis is a rare cause of prosthetic or native valve endocarditis [162–170]. Intravenous drug use is the typical risk factor [170–172]. Endocarditis occurs principally on or around prosthetic valves and can cause aortic thrombosis [167]. Osteomyelitis usually occurs after traumatic inoculation or surgical intervention (eg, tibial pin placement, anterior cruciate ligament repair) [171–173]. Authors have reported osteomyelitis of the tibia, cuboid, calcaneus, femur, humerus, scapula, metacarpals, phalanges, and sternum [174–177]. Hematogenous osteomyelitis is extremely rare [177]. Also rare is involvement of the peritoneal cavity by Zygomycetes in patients undergoing continuous ambulatory peritoneal dialysis [178, 179].
Peritonitis tends to be slowly progressive, although the attributable mortality rate in patients receiving delayed or inappropriate treatment has been as high as 60% [180, 181]. In all cases of catheter-related mucormycosis, prompt removal of the catheter and use of systemic antifungal therapy for several weeks are essential. Although rare, renal mucormycosis should be suspected in any immunocompromised patient who presents with hematuria, flank pain, and unexplained anuric renal failure. Isolated renal mucormycosis has occurred in intravenous drug users as well as renal transplant recipients in developing countries with warm climates such as India, Egypt, Saudi Arabia, Kuwait, and Singapore [53, 118, 182]. Another rare manifestation of mucormycosis is brain involvement (typically in the basal ganglia) without rhino-orbital involvement in patients with leukemia and intravenous drug abusers [160]. Finally, isolated mucormycosis in the mastoid, oral mucosa, bone [173], the bladder, the trachea [183], the mediastinum [123, 184], or the ear is rare.
CONCLUSION
Mucormycosis is a rare but emerging fungal infection with a high mortality rate. Most of the existing epidemiological studies of mucormycosis are retrospective and limited. The literature contains few prospective, population-wide studies of it. Available studies typically took place at institutions with specific populations at risk for mucormycosis (patients with HM and recipients of transplants). The incidence of mucormycosis seems to be increasing in leukemic patients and stem cell transplant recipients chronically exposed to Aspergillus-active agents, although the generalizability of this observation is controversial. Despite the diabetes epidemic in developed countries, the incidence of mucormycosis in diabetics may be decreasing. In contrast, in developing countries, uncontrolled diabetes mellitus and trauma are the most common risk factors for mucormycosis. More representative data on specific groups of patients (eg, leukemic patients, transplant recipients, diabetics) are needed for better evaluation of the infection. Well-organized global registries are needed to estimate the burden of mucormycosis. The pleiotropic clinical manifestations and elusive presentation of mucormycosis often delay diagnosis, with resultant poor outcomes. A high index of suspicion for mucormycosis based on appropriate risk stratification and improved laboratory diagnosis are important for improving the natural history of this devastating infection.
Notes
Supplement sponsorship.
This article was published as part of a supplement entitled “Advances Against Mucormycosis: A Tribute to the Memory and Courage of Hank Schueler,” sponsored by the Henry Schueler 41&9 Foundation.
Potential conflicts of interest.
G. P. has received grant support from Gilead, Pfizer, Schering Plough, Aventis, and Merck Sharp & Dohme (MSD); has acted as a paid consultant to Janssen Cilag, Gilead, Astellas, and Schering Plough; and is a member of the speakers bureaus for Gilead, Schering Plough, and MSD. E.R. has received grant support from Pfizer, Gilead, Enzon, Schering, and Wyeth; has acted as a paid consultant to Schering, Gilead, Astellas, and Pfizer; and is a member of the speakers bureaus for Gilead, Cephalon, Pfizer, Wyeth, Schering, Merck, Aventis, and Astellas. T. J. W. has received grant support from Novartis, Astellas and is a consultant for Trius, iCo, Sigma Tau, and Draius. D. P. K. has received research support and honoraria from Merck, Pfizer, Fujisawa Pharmaceutical, and Enzon Pharmaceuticals and serves on the advisory boards for Merck and Schering-Plough Research Institute. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.




![Most frequent agents of mucormycosis. Data from 5 studies [16, 27, 28, 33, 38].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/cid/54/suppl_1/10.1093_cid_cir866/1/m_cidcir866f01_3c.gif?Expires=1716383301&Signature=DIOS5S-a7WKtuBHNqznZSk45sK6XFWxmv~hqXusweanV2fc3MDEhKoJEEXgszXYxHNBIzlyeIltJHuIFDhNF9rC3W4G8CdNWO8BBsPUR3yIAkgfg4Hw7w-aszSaHNopGpZ7ij9XTsHbwbRuEeKz1DqjqTXu-YzxUt5DCzwL9kmwnkdwcEyC3RJ4arpWZ3ikmJnQtd0E3fa1YA50QDaTPDBjvIvi8BncRfwsKTj~oQBIghOHSRJMCiQdQ8fTZwWfQqtuZjK1~qmQGnLCd6r5UMUGJUOvNLVCC-24SOl~~DdNg9SsbSWHS5WGm5CW5cps08oKSzqWUl~45lEwUj15RgA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

Comments