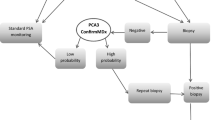
Abstract
BACKGROUND: Prostate-specific antigen (PSA) levels between 4.0 to 10.0 ng/ml have poor specificity in prostate cancer screening, leading to unnecessary biopsies.
OBJECTIVE: To determine whether the free-to-total PSA ratio (F/T PSA) improved the diagnostic accuracy of these nonspecific PSA levels.
MEASUREMENTS AND MAIN RESULTS: Medline was searched from 1986 to 1997. Additional studies were identified from article bibliographies and by searching urology journals. Two investigators independently identified English-language studies providing F/T PSA ratio test-operating characteristics data on ≧10 cancer patients with PSA values between 2.0 and 10.0 ng/ml. Twenty-one of 90 retrieved studies met selection criteria. Two investigators independently extracted data on methodology and diagnostic performance. Investigator-selected cut points for the optimal F/T PSA ratio had a median likelihood ratio of 1.76 (interquartile range, 1.40 to 2.11) for a positive test and 0.27 (0.20 to 0.40) for a negative test. Assuming a 25% pretest probability of cancer, the posttest probabilities were 37% following a positive test and 8% following a negative test. The summary receiver operating characteristic curve showed that maintaining test sensitivity above 90% was associated with false positive rates of 60% to 90%. Methodologic problems limited the validity and generalizability of the literature.
CONCLUSIONS: A negative test reduced the posttest probability of cancer to approximately 10%. However, patients may find that this probability is not low enough to avoid undergoing prostate biopsy. The optimal F/T PSA ratio cut point and precise estimates for test specificity still need to be determined.
Similar content being viewed by others
References
Mettlin C, Jones G, Averette H, Gusberg SB, Murphy GP. Defining and updating the American Cancer Society guidelines for the cancer related check-up: prostate and endometrial cancers. CA Cancer J Clin. 1993;43:42–6.
American Urological Association. Early detection of prostate cancer and use of transrectal ultrasound. American Urological Association 1992 Policy Statement Book. Baltimore, Md: Williams & Wilkins; 1992.
PDQ. (Physician Data Query) [database online]. Bethesda, Md: National Cancer Institute: 1984 — [updated 9/99]. Screening for prostate cancer. Available from: National Cancer Institute; National Library of Medicine, Bethesda, Md: CDP Technologies, Inc., New York, NY; Lexis-Nexis, Miamisburg, Ohio.
American College of Physicians. Screening for prostate cancer. Ann Intern Med. 1997;126:480–4.
U.S. Preventive Services Task Force. Guide to clinical preventive services. 2nd ed. Baltimore: Williams & Wilkins; 1996.
Catalona WJ, Richie JP, Ahamann FR, et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multicenter clinical trial of 6,630 men. J Urol. 1994;151:1283–90.
Carter HB, Pearson JD, Metter EJ, et al. Longitudinal evaluation of prostate-specific antigen levels in men with and without prostate disease. JAMA. 1992;267:2215–20.
Benson MC, Whang IS, Olsson CA, McMahon DJ, Cooner WH. The use of prostate specific antigen density to enhance the predictive value of intermediate levels of serum prostate specific antigen. J Urol. 1992;147:817–21.
Oesterling JE, Jacobsen SJ, Chute CG, et al. Serum prostate-specific antigen in a community-based population of healthy men. Establishment of age-specific reference ranges. JAMA. 1993;270:860–4.
Lilja H, Christensson A, Dahlén U, et al. Prostate-specific antigen in serum occurs predominantly in complex with α1-antichymotrypsin. Clin Chem. 1991;37:1618–25.
Stenman UH, Leinonen J, Alfthan H, Rannikko S, Tuhkanen K, Alfthan O. A complex between prostate-specific antigen and α1-antichymotrypsin is the major form of prostate-specific antigen in serum of patients with prostatic cancer: assay of the complex improves clinical sensitivity for cancer. Cancer Res. 1991;51:222–6.
Christensson A, Laurell CB, Lilja H. Enzymatic activity of prostate-specific antigen and its reactions with extracellular serine proteinase inhibitors. Eur J Biochem. 1990;194:755–63.
McCormack RT, Rittenhouse HG, Finlay JA, et al. Molecular forms of prostate-specific antigen and the human kallikrein gene family: a new era. Urology. 1995;45:729–44.
Lilja H. Regulation of the enzymatic activity of prostate-specific antigen and its reactions with extracellular protease inhibitors in prostate cancer. Scand J Clin Lab Invest Suppl. 1995;220:47–56.
Leinonen J, Lövgren T, Vornanen T, Stenman UH. Double-label time-resolved immunofluorometric assay of prostate-specific antigen and of its complex with α1-antichymotrypsin. Clin Chem. 1993;39:2098–103.
Christensson A, Björk T, Nilsson O, et al. Serum prostate specific antigen complexed to α1-antichymotrypsin as an indicator of prostate cancer. J Urol. 1993;150:100–5.
Abrahamsson PA, Lilja H, Oesterling JE. Molecular forms of serum prostate-specific antigen. The clinical value of percent free prostate-specific antigen. Urol Clin North Am. 1997;24:353–65.
Vessella RL, Lange PH. Issues in the assessment of prostate-specific antigen immunoassays. An update. Urol Clin North Am. 1997;24:261–8.
Colberg JW, Smith DS, Catalona WJ. Prevalence and pathological extent of prostate cancer in men with prostate specific antigen levels of 2.9 to 4.0 ng/ml. J Urol. 1993;149:507–9.
Gann PH, Hennekens CH, Stampfer MJ. A prospective evaluation of plasma prostate-specific antigen for detection of prostatic cancer. JAMA. 1995;273:289–94.
Vashi AR, Oesterling JE. Percent free prostate-specific antigen: entering a new era in the detection of prostate cancer. Mayo Clin Proc. 1997;72:337–44.
Catalona WJ. Clinical utility of measurements of free and total prostate-specific antigen (PSA): a review. Prostate Suppl. 1996;7:64–9.
U.S. Food and Drug Administration. Center for Devices and Radiological Health. Premarket Approval Decisions for March 1998. Available at: http://www.fda.gov:80/cdrh/pmamar98.html.
Catalona WJ, Partin AW, Slawin KM, et al. A multicenter clinical trial evaluation of free PSA in the differentiation of prostate cancer from benign disease. J Urol. 1997;157(suppl):111.
Ransohoff DF, Feinstein AR. Problems of spectrum and bias in evaluating the efficacy of diagnostic tests. New Engl J Med. 1978;299:926–30.
Jaeschke R, Guyatt G, Sackett DL. Users’ guides to the medical literature. III. How to use an article about a diagnostic test. A. Are the results of the study valid? JAMA. 1994;271:389–91.
Jaeschke R, Guyatt GH, Sackett DL. Users’ guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? JAMA. 1994;271:703–7.
Mulrow CD, Linn WD, Gaul MK, Pugh JA. Assessing quality of a diagnostic test evaluation. J Gen Intern Med. 1989;4:288–95.
Irwig L, Tosteson ANA, Gatsonis C, et al. Guidelines for meta-analyses evaluating diagnostic tests. Ann Intern Med. 1994;120:667–76.
Reid MC, Lachs MS, Feinstein AR. Use of methodlogical standards in diagnostic test research. Getting better but still not good. JAMA. 1995;274:645–51.
Arcangeli CG, Smith DS, Ratliff TL, Catalona WJ. Stability of serum total and free prostate specific antigen under varying storage intervals and temperatures. J Urol. 1997;158:2182–7.
Woodrum D, French C, Shamel LB. Stability of free prostate-specific antigen in serum samples under a variety of sample collection and sample storage conditions. Urology. 1996;48:33–9.
Jacobsen SJ, Lija H, Klee GG, Wright GL, Jr., Pettersson K, Oesterling JE. Comparability of the Tandem-R and IMx assays for the measurement of serum prostate-specific antigen. Urology. 1994;44:512–8.
Pienta KJ, Esper PS. Risk factors for prostate cancer. Ann Intern Med. 1993;118:793–803.
Marley GM, Miller MC, Kattan MW, et al. Free and complexed prostate-specific antigen serum ratios to predict probability of primary prostate cancer and benign prostatic hyperplasia. Urology. 1996;48:16–22.
Stamey TA. Progress in standardization of immunoassays for prostate-specific antigen. Urol Clin North Am. 1997;24:269–73.
Sackett DL, Haynes RB, Guyatt GH, Tugwell P. Clinical Epidemiology. A basic science for clinical medicine. 2nd ed. Boston: Little, Brown; 1991.
Peirce JC, Cornell RG. Integrating stratum-specific likelihood ratios with the analysis of ROC curves. Med Decis Making. 1993;13:141–51.
Brawer MK, Chetner MP, Beatie J, Buchner DM, Vessella RL, Lange PH. Screening for prostatic carcinoma with prostate specific antigen. J Urol. 1992;147:841–5.
Moses LE, Shapiro D, Littenberg B. Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med. 1993;12:1293–316.
Littenberg B, Moses LE. Estimating diagnostic accuracy from multiple conflicting reports: a new meta-analytic method. Med Decis Making. 1993;13:313–21.
SAS® Language: Reference. Version 6. Cary, NC: SAS Institute, Inc.; 1990.
Elgamal AA, Cornillie FJ, Van Poppel HP, Van de Voorde WM, McCabe R, Baert LV. Free-to-total prostate specific antigen ratio as a single test for detection of significant stage T1c prostate cancer. J Urol. 1996;156:1042–9.
Froschermaier SE, Pilarsky CP, Wirth MP. Clinical significance of the determination of noncomplexed prostate-specific antigen as a marker for prostate carcinoma. Urology. 1996;47:525–8.
Junker R, Brandt B, Zechel C, Assmann G. Comparison of prostate-specific antigen (PSA) measured by four combinations of free PSA and total PSA assays. Clin Chem. 1997;43:1588–94.
Mitrunen K, Pettersson K, Piironen T, Björk T, Lilja H, Lövgren T. Dual-label one-step immunoassay for simultaneous measurement of free and total prostate-specific antigen concentrations and ratios in serum. Clin Chem. 1995;41:1115–20.
Reissigl A, Klocker H, Pointner J, Ennemoser O, Falk M, Bartsch G. Improvement of prostate cancer screening by determination of the ratio free/total PSA in addition to PSA levels. Prostate. 1997;30:243–7.
Riccardo B, Alberino D, Fabrizio T, et al. Free to total prostatic specific antigen ratio as a new diagnostic tool in prostatic carcinoma. Anticancer Res. 1997;17:1297–302.
Stephan C, Lein M, Jung K, Schnorr D, Loening SA. The influence of prostate volume on the ratio of free to total prostate specific antigen in serum of patients with prostate cancer and benign prostate hyperplasia. Cancer. 1997;79:104–9.
Tarle M, Kraljic I. Free and total serum PSA values in patients with prostatic intraepithelial neoplasia (PIN), prostate cancer and BPH. Is F/T PSA a potential probe for dormant and manifest cancer? Anticancer Res. 1997;17:1531–4.
Thiel RP, Oesterling JE, Wojno KJ, et al. Multicenter comparison of the diagnostic performance of free prostate-specific antigen. Urology. 1996;48:45–50.
Wolff JM, Borchers H, Effert PJ, Habib FK, Jakse G. Free-to-total prostate-specific antigen serum concentrations in patients with prostate cancer and benign prostatic hyperplasia. Br J Urol. 1996;78:409–13.
Morote J, Raventós CX, Lorente JA, et al. Measurement of free PSA in the diagnosis and staging of prostate cancer. Int J Cancer. 1997;71:756–9.
Murphy GP, Barren RJ, Erickson SJ, et al. Evaluation and comparison of two new prostate carcinoma markers. Free-prostate specific antigen and prostate specific membrane antigen. Cancer. 1996;78:809–18.
Prestigiacomo AF, Stamey TA. Clinical usefulness of free and complexed PSA. Scand J Clin Lab Invest Suppl. 1995;221:32–4.
Chen YT, Luderer AA, Thiel RP, Carlson G, Cuny CL, Soriano TF. Using proportions of free to total prostate-specific antigen, age, and total prostate-specific antigen to predict the probability of prostate cancer. Urology. 1996;47:518–24.
Higashihara E, Nutahara K, Kojima M, et al. Significance of serum free prostate specific antigen in the screening of prostate cancer. J Urol. 1996;156:1964–8.
Morgan TO, McLeod DG, Leifer ES, Moul JW, Murphy GP. Prospective use of free PSA to avoid repeat prostate biopsies in men with elevated total PSA. Prostate Suppl. 1996;7:58–63.
Akdas A, Cevik I, Tarcan T, Turkeri L, Dalaman G, Emerk K. The role of free prostate-specific antigen in the diagnosis of prostate cancer. Brit J Urol. 1997;79:920–3.
Auvinen A, Tammela T, Stenman UH, et al. Screening for prostate cancer using serum prostate-specific antigen: a randomised, population-based pilot study in Finland. Br J Cancer. 1996;74:568–72.
Correale M, Pagliarulo A, Donatuti G, et al. Preliminary clinical evaluation of free/total PSA ratio by the IMMULITE system. Int J Biol Markers. 1996;11:24–8.
Reissigl A, Pointner J, Horninger W, et al. Comparison of different prostate-specific antigen cutpoints for early detection of prostate cancer: results of a large screening study. Urology. 1995;46:662–5.
Van Cangh PJ, De Nayer P, Sauvage P, et al. Free to total prostate-specific antigen (PSA) ratio is superior to total-PSA in differentiating benign prostate hypertrophy from prostate cancer. Prostate Suppl. 1996;7:30–4.
Bangma CH, Kranse R, Blijenberg BG, Schröder FH. The value of screening tests in the detection of prostate cancer. Part II: Retrospective analysis of free/total prostate-specific analysis ratio, age-specific reference ranges, and PSA density. Urology. 1995;46:779–84.
Bangma CH, Kranse R, Blijenberg BG, Schröder FH. The value of screening tests in the detection of prostate cancer. Part I: Results of a retrospective evaluation of 1726 men. Urology. 1995;46:773–8.
Bangma CH, Kranse R, Blijenberg BG, Schröder FH. Free and total prostate-specific antigen in a screened population. Br J Urol. 1997;79:756–62.
Morgan TO, McLeod DG, Leifer ES, Murphy GP, Moul JW. Prospective use of free prostate-specific antigen to avoid repeat prostate biopsies in men with elevated total prostate-specific antigen. Urology. 1996;48:76–80.
Demura T, Shinohara N, Tanaka M, et al. The proportion of free to total prostate specific antigen: a method of detecting prostate carcinoma. Cancer. 1996;77:1137–43.
Demura T, Watarai T, Togashi M, Hirano T, Ohashi N, Koyanagi T. Measurement of prostate specific antigen and γ-seminoprotein ratio: a new means of distinguishing benign prostatic hyperplasia and prostate cancer. J Urol. 1993;150:1740–5.
Kuriyama M, Takeuchi T, Shinoda I, Okana M, Nishiura T. Clinical evaluation of γ-seminoprotein in prostate cancer. Prostate. 1986;8:301–11.
Stenman UH, Hakama M, Knekt P, Aromaa A, Teppo L, Leinonen J. Serum concentrations of prostate specific antigen and its complex with α1-antichymotrypsin before diagnosis of prostate cancer. Lancet. 1994;344:1594–8.
Prestigiacomo AF, Stamey TA. Can free and total prostate specific antigen and prostatic volume distinguish between men with negative and positive systematic ultrasound guided prostate biopsies? J Urol. 1997;157:189–94.
Alivizatos G, Deliveliotis C, Mitropoulos D, et al. Does free to total ratio of prostate-specific antigen alter decision-making on prostatic biopsy? Urology. 1996;48:71–5.
Bangma CH, Rietbergen JBW, Kranse R, Blijenberg BG, Petterson K, Schröder FH. The free-to-total prostate specific antigen ratio improves the specificity of prostate specific antigen in screening for prostate cancer in the general population. J Urol. 1997;157:2191–6.
Björk T, Piironen T, Pettersson K, et al. Comparison of analysis of the different prostate-specific antigen forms in serum for detection of clinically localized prostate cancer. Urology. 1996;48:882–8.
Catalona WJ, Smith DS, Wolfert RL, et al. Evaluation of percent-age of free serum prostate-specific antigen to improve specificity of prostate cancer screening. JAMA. 1995;274:1214–20.
Catalona WJ, Partin AW, Slawin KM, et al. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease. A prospective multicenter clinical trial. JAMA. 1998;279:1542–7.
Egawa S, Soh S, Ohori M, et al. The ratio of free to total serum prostate specific antigen and its use in differential diagnosis of prostate carcinoma in Japan. Cancer. 1997;79:90–8.
Filella X, Alcover J, Molina R, et al. Clinical usefulness of free PSA fraction as an indicator of prostate cancer. Int J Cancer. 1995;63:780–4.
Jung K, Stephan C, Lein M, et al. Analytical performance and clinical validity of two free prostate-specific antigen assays compared. Clin Chem. 1996;42:1026–33.
Luderer AA, Chen YT, Soriano TF, et al. Measurement of the proportion of free to total prostate-specific antigen improves diagnostic performance of prostate-specific antigen in the diagnostic gray zone of total prostate-specific antigen. Urology. 1995;46:187–94.
Mione R, Aimo G, Bombardieri E, et al. Preliminary results of clinical evaluation of the free/total prostate-specific antigen ratio in a multicentric study. Tumori. 1996;82:543–9.
Partin AW, Catalona WJ, Southwick PC, Subong ENP, Gasior GH, Chan DW. Analysis of percent free prostate-specific antigen (PSA) for prostate cancer detection: influence of total PSA, prostate volume, and age. Urology. 1996;48:55–61.
Prestigiacomo AF, Lilja HJ, Pettersson K, Wolfert RL, Stamey TA. A comparison of the free fraction of serum prostate specific antigen in men with benign and cancerous prostates: the best case scenario. J Urol. 1996;156:350–4.
Roehrborn CG, Gregory A, McConnell JD, Sagalowsky AI, Wians FH Jr. Comparison of three assays for total serum prostate-specific antigen and percentage of free prostate-specific antigen in predicting prostate histology. Urology. 1996;48:23–32.
Van Cangh PJ, De Nayer P, De Vischer L, et al. Free to total prostate-specific antigen (PSA) ratio improves the discrimination between prostate cancer and benign prostatic hyperplasia (BPH) in the diagnostic gray zone of 1.8 to 10 ng/mL total PSA. Urology. 1996;48:67–70.
Vashi AR, Wojno KJ, Henricks W, et al. Determination of the “reflex range” and appropriate cutpoints for percent free prostate-specific antigen in 413 men referred for prostatic evaluation using the AxSYM system. Urology. 1997;49:19–27.
Wang TJ, Hill TM, Sokoloff RL, Frankenne F, Rittenhouse HG, Wolfert RL. Dual monoclonal antibody immunoassay for free prostate-specific antigen. Prostate. 1996;28:10–6.
Reissigl A, Klocker H, Pointner J, et al. Usefulness of the ratio free/total prostate-specific antigen in addition to total PSA levels in prostate cancer screening. Urology. 1996;48:62–6.
Toubert ME, Guillet J, Chiron M, et al. Percentage of free serum prostate-specific antigen: a new tool in the early diagnosis of prostatic cancer. Eur J Cancer. 1996;32A:2088–93.
Catalona WJ, Smith DS, Ornstein DK. Prostate cancer detection in men with serum PSA concentrations of 2.6 to 4.0 ng/ml and benign prostate examination. Enhancement of specificity with free PSA measurements. JAMA. 1997;277:1452–5.
Schröder FH, van der Cruijsen-Koeter I, de Koning HJ, Vis AN, Hoedemaker RF, Kranse R. Prostate cancer detection at low prostate specific antigen. J Urol. 2000;163:806–12.
Lachs MS, Nachamkin I, Edelstein PH, Goldman J, Feinstein AR, Schwartz JS. Spectrum bias in the evaluation of diagnostic tests: lessons from the rapid dipstick test for urinary tract infection. Ann Intern Med. 1992;117:135–40.
Stroumbakis N, Cookson MS, Reuter VE, Fair WR. Clinical significance of repeat sextant biopsies in prostate cancer patients. Urology. 1997;49(suppl):113–8.
Ellis WJ, Brawer MK. Repeat prostate needle biopsy: who needs it? J Urol. 1995;153:1496–8.
Buck AA, Gart JJ. Comparison of a screening test and a reference test in epidemiologic studies. Indices of agreement and their relation to prevalence. Am J Epidemiol. 1966;83:586–92.
Boyko EJ, Alderman BW, Baron AE. Reference test errors bias the evaluation of diagnostic tests for ischemic heart disease. J Gen Intern Med. 1988;3:476–81.
Nixon RG, Petteway JC, Meyer GE, Brawer MK. Comparison of three investigative assays for the free form of prostate-specific antigen. J Urol. 1997;157(suppl):255.
Ornstein DK, Smith DS, Rao GS, Basler JW, Ratliff TL, Catalona WJ. Biological variation of total, free and percent free serum prostate specific antigen levels in screening volunteers. J Urol. 1997;157:2179–82.
Nixon RG, Lilly JD, Liedtke RJ, Batjer JD. Variation of free and total prostate-specific antigen levels: The effect on percent free/total prostate-specific antigen. Arch Pathol Lab Med. 1997;121:385–91.
Brawer MK. Prostate-specific antigen: current status. CA Cancer J Clin. 1999;49:264–81.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the VA Medical Center, Albuquerque, NM.
Rights and permissions
About this article
Cite this article
Hoffman, R.M., Clanon, D.L., Littenberg, B. et al. Using the free-to-total prostate-specific antigen ratio to detect prostate cancer in men with nonspecific elevations of prostate-specific antigen levels. J GEN INTERN MED 15, 739–748 (2000). https://doi.org/10.1046/j.1525-1497.2000.90907.x
Issue Date:
DOI: https://doi.org/10.1046/j.1525-1497.2000.90907.x