Abstract
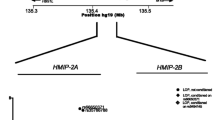
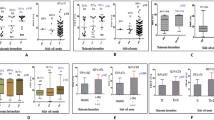
β-Thalassemia/HbE disease is clinically variable. In searching for genetic factors modifying the disease severity, patients were selected based on their disease severities, and a genome-wide association study (GWAS) was performed. Genotyping was conducted with the Illumina Human 610-Quad BeadChips array using DNAs from 618 Thai β0-thalassemia/HbE patients who were classified as 383 severe and 235 mild phenotypes by a validated scoring system. Twenty-three SNPs in three independent genes/regions were identified as being significantly associated with the disease severity. The highest association was observed with SNPs in the β-globin gene cluster (chr.11p15), and rs2071348 of the HBBP1 gene revealed the most significant association [P = 2.96 × 10−13, odds ratio (OR) = 4.33 (95% confidence interval (CI), 2.74–6.84)]. The second was identified in the intergenic region between the HBS1L and MYB genes (chr.6q23), among which rs9376092 was the most significant [P = 2.36 × 10−10, OR = 3.07 (95% CI, 2.16–4.38)]. The third region was located in the BCL11A gene (chr.2p16.1), and rs766432 showed the most significant association [P = 5.87 × 10−10, OR = 3.06 (95% CI, 2.15–4.37)]. All three loci were replicated in an independent cohort of 174 Indonesian patients. The associations to fetal hemoglobin levels were also observed with SNPs on these three regions. Our data indicate that several genetic loci act in concert to influence HbF levels of β0-thalassemia/HbE patients. This study revealed that all the three reported loci and the α-globin gene locus are the best and common predictors of the disease severity in β-thalassemia.



Similar content being viewed by others
References
Calzolari R, McMorrow T, Yannoutsos N, Langeveld A, Grosveld F (1999) Deletion of a region that is a candidate for the difference between the deletion forms of hereditary persistence of fetal hemoglobin and δβ-thalassemia affects β- but not γ-globin gene expression. EMBO J 18:949–958
Chong SS, Boehm CD, Cutting GR, Higgs DR (2000) Simplified multiplex-PCR diagnosis of common Southeast Asian deletional determinants of α-thalassemia. Clin Chem 46:1692–1695
Creary LE, McKenzie CA, Menzel S, Hanchard NA, Taylor V, Hambleton I, Spector TD, Forrester TE, Thein SL (2009) Ethnic differences in F cell levels in Jamaica: a potential tool for identifying new genetic loci controlling fetal haemoglobin. Br J Haematol 144:954–960
Dostie J, Richmond TA, Arnaout RA, Selzer RR, Lee WL, Honan TA, Rubio ED, Krumm A, Lamb J, Nusbaum C, Green RD, Dekker J (2006) Chromosome conformation capture carbon copy (5C): a massively parallel solution for mapping interactions between genomic elements. Genome Res 16:1299–1309
Flint J, Harding RM, Boyce AJ, Clegg JB (1998) The population genetics of the haemoglobinopathies. In: Higgs DR, Weatherall DJ (eds) Baillière’s Clinical Haematology; ‘Haemoglobinopathies’, vol 11. W.B. Saunders, London, pp 1–51
Gribnau J, Diderich K, Pruzina S, Calzolari R, Fraser P (2000) Intergenic transcription and developmental remodeling of chromatin subdomains in the human β-globin locus. Mol Cell 5:377–386
Lettre G, Sankaran VG, Bezerra MA, Araujo AS, Uda M, Sanna S, Cao A, Schlessinger D, Costa FF, Hirschhorn JN, Orkin SH (2008) DNA polymorphisms at the BCL11A, HBS1L-MYB, and β-globin loci associate with fetal hemoglobin levels and pain crises in sickle cell disease. Proc Natl Acad Sci USA 105:11869–11874
Ma Q, Abel K, Sripichai O, Whitacre J, Angkachatchai V, Makarasara W, Winichagoon P, Fucharoen S, Braun A, Farrer LA (2007) β-globin gene cluster polymorphisms are strongly associated with severity of HbE/β0-thalassemia. Clin Genet 72:497–505
Menzel S, Garner C, Gut I, Matsuda F, Yamaguchi M, Heath S, Foglio M, Zelenika D, Boland A, Rooks H, Best S, Spector TD, Farrall M, Lathrop M, Thein SL (2007a) A QTL influencing F cell production maps to a gene encoding a zinc-finger protein on chromosome 2p15. Nat Genet 39:1197–1199
Menzel S, Jiang J, Silver N, Gallagher J, Cunningham J, Surdulescu G, Lathrop M, Farrall M, Spector TD, Thein SL (2007b) The HBS1L-MYB intergenic region on chromosome 6q23.3 influences erythrocyte, platelet, and monocyte counts in humans. Blood 110:3624–3626
Michelson AM (2008) Developmental biology: from genetic association to genetic switch. Science 322:1803–1804
Miles J, Mitchell JA, Chakalova L, Goyenechea B, Osborne CS, O’Neill L, Tanimoto K, Engel JD, Fraser P (2007) Intergenic transcription, cell-cycle and the developmentally regulated epigenetic profile of the human β-globin locus. PLoS ONE 2:e630
Ohnishi Y, Tanaka T, Ozaki K, Yamada R, Suzuki H, Nakamura Y (2001) A high-throughput SNP typing system for genome-wide association studies. J Hum Genet 46:471–477
Phadke SR, Agarwal S (2003) Phenotype score to grade the severity of thalassemia intermedia. Indian J Pediatr 70:477–481
Premawardhena A, Fisher CA, Olivieri NF, de Silva S, Arambepola M, Perera W, O’Donnell A, Peto TE, Viprakasit V, Merson L, Muraca G, Weatherall DJ (2005) Haemoglobin E β-thalassaemia in Sri Lanka. Lancet 366:1467–1470
Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D (2006) Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38:904–909
Quek Lynn, Thein SL (2007) Molecular therapies in β-thalassaemia. Br J Haematol 136:353–365
Rooks H, Bergounioux J, Game L, Close JP, Osborne C, Best S, Senior T, Height S, Thompson R, Hadzic N, Fraser P, Bolton-Maggs P, Thein SL (2005) Heterogeneity of the εγδβ-thalassaemias: characterization of three novel English deletions. Br J Haematol 128:722–729
Sankaran VG, Menne TF, Xu J, Akie TE, Lettre G, Van Handel B, Mikkola HK, Hirschhorn JN, Cantor AB, Orkin SH (2008) Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science 322:1839–1842
Sedgewick AE, Timofeev N, Sebastiani P, So JC, Ma ES, Chan LC, Fucharoen G, Fucharoen S, Barbosa CG, Vardarajan BN, Farrer LA, Baldwin CT, Steinberg MH, Chui DH (2008) BCL11A is a major HbF quantitative trait locus in three different populations with β-hemoglobinopathies. Blood Cells Mol Dis 41:255–258
Sripichai O, Makarasara W, Munkongdee T, Kumkhaek C, Nuchprayoon I, Chuansumrit A, Chuncharunee S, Chantrakoon N, Boonmongkol P, Winichagoon P, Fucharoen S (2008a) A scoring system for the classification of β-thalassemia/Hb E disease severity. Am J Hematol 83:482–484
Sripichai O, Munkongdee T, Kumkhaek C, Svasti S, Winichagoon P, Fucharoen S (2008b) Coinheritance of the different copy numbers of α-globin gene modifies severity of β-thalassemia/Hb E disease. Ann Hematol 87:375–379
Sutton M, Bouhassira EE, Nagel RL (1989) Polymerase chain reaction amplification applied to the determination of β-like globin gene cluster haplotypes. Am J Hematol 32:66–69
Thein SL (2005) Genetic modifiers of β-thalassemia. Haematologica 90:649–660
Thein SL, Menzel S, Peng X, Best S, Jiang J, Close J, Silver N, Gerovasilli A, Ping C, Yamaguchi M, Wahlberg K, Ulug P, Spector TD, Garner C, Matsuda F, Farrall M, Lathrop M (2007) Intergenic variants of HBS1L-MYB are responsible for a major quantitative trait locus on chromosome 6q23 influencing fetal hemoglobin levels in adults. Proc Natl Acad Sci USA 104:11346–11351
Wahlberg K, Jiang J, Rooks H, Jawaid K, Matsuda F, Yamaguchi M, Lathrop M, Thein SL, Best S (2009) The HBS1L-MYB intergenic interval associated with elevated HbF levels shows characteristics of a distal regulatory region in erythroid cells. Blood 114:1254–1262
Weatherall DJ (1998) Hemoglobin E-β-thalassemia: an increasingly common disease with some diagnostic pitfalls. J Pediatr 132:765–767
Winichagoon P, Thonglairoam V, Fucharoen S, Wilairat P, Fukumaki Y, Wasi P (1993) Severity differences in β-thalassaemia/haemoglobin E syndromes: implication of genetic factors. Br J Haematol 83:633–639
Winichagoon P, Saechan V, Sripanich R, Nopparatana C, Kanokpongsakdi S, Maggio A, Fucharoen S (1999) Prenatal diagnosis of β-thalassaemia by reverse dot-blot hybridization. Prenat Diagn 19:428–435
Winichagoon P, Fucharoen S, Chen P, Wasi P (2000) Genetic factors affecting clinical severity in β-thalassemia syndromes. J Pediatr Hematol Oncol 22:573–580
Acknowledgments
We thank all of the patients who participated in this study, and we thank Dr. Sumonmaln Klamchuen, Nakorn Pathom Hospital; Dr. Su-on Chainunsamit, Khon Kaen Hospital; Dr. Issarang Nuchprayoon, Chulalongkorn Hospital, Dr. Leelawan Wiboonmongkol, Rachaburi Hospital; Dr. Ampaiwan Chuansumrit, Ramathibodi Hospital, Thailand for their kind support in contacting subjects. We thank Siti Ayu Putriasih from Department of Child Health Medical Faculty, University of Indonesia, Cipto Mangunkusumo National Hospital. We thank Budi Amarta Putra, Dessy Handayani, Felix Liaw from Medical Faculty University of Indonesia. We thank Ita Margaretha Nainggolan, Mewahyu Dewi, and Arleen Nugraha Suryatenggara from Eijkman Institute for Molecular Biology, Indonesia for their kind support in contacting subjects for replication study in the Indonesian population. We acknowledge the Thailand Research Fund for encouraging the study. This work was mainly supported by the DMSc-RIKEN collaboration and the National Center for Genetic Engineering and Biotechnology (BIOTEC), Thailand. Additional support was provided by the Higher Education Commission, the Siriraj Graduate Thesis Scholarship and the Medical Scholar Program, Mahidol University, Thailand.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nuinoon, M., Makarasara, W., Mushiroda, T. et al. A genome-wide association identified the common genetic variants influence disease severity in β0-thalassemia/hemoglobin E. Hum Genet 127, 303–314 (2010). https://doi.org/10.1007/s00439-009-0770-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-009-0770-2