Abstract
Purpose
The use of noninvasive ventilation (NIV) to facilitate discontinuation of mechanical ventilation in patients with acute hypoxemic respiratory failure (hypoxemic ARF) has never been explored. This pilot study aims to assess the feasibility of early extubation followed by immediate NIV, compared conventional weaning, in patients with resolving hypoxemic ARF.
Methods
Twenty consecutive hypoxemic patients were randomly assigned to receive either conventional weaning or NIV. The changes in arterial blood gases and respiratory rate were compared between the two groups at 1, 12, 24 and 48 h. Differences in the rate of extubation failure, ICU and hospital mortality, number of invasive-ventilation-free-days at day 28, septic complications, number of tracheotomies, days and rates of continuous intravenous sedation, and ICU length of stay were also determined.
Results
No patient interrupted the study protocol. Arterial blood gases were similar during invasive mechanical ventilation, 1 h after NIV application following extubation, and after 12, 24 and 48 h. Respiratory rate was higher after 1 h in the NIV group, but no different after 12, 24 and 48 h. The number of invasive-ventilation-free-days at day 28 was 20 ± 8 (min = 0, max = 25) days in the treatment group and 10 ± 9 (min = 0, max = 25) days in the control group (p = 0.014). The rate of extubation failure, ICU and hospital mortality, tracheotomies, septic complications, days and rates of continuous sedation, and ICU length of stay were not significantly different between the two groups.
Conclusions
In a highly experienced centre NIV may be used to facilitate discontinuation of mechanical ventilation in selected patients with resolving hypoxemic ARF.
Similar content being viewed by others
Introduction
The delivery of mechanical ventilation through an endotracheal tube is a life-saving treatment for patients with acute hypoxemic respiratory failure (hypoxemic ARF), but it is affected by serious complications [1]. While some of these, such as the need for heavy sedation [2], are remarkably reduced by replacing controlled mechanical ventilation with forms of partial ventilatory support, ventilator-associated pneumonia (VAP) is consequent to the endotracheal tube regardless of the mode of ventilation adopted [3].
Noninvasive ventilation (NIV) is effective in improving gas exchange while reducing dyspnoea [4–6] and inspiratory effort in patients with either hypoxemic [5] and hypercapnic ARF [7] and averts the risk secondary to endotracheal intubation. NIV preserves airway defence mechanisms, speech, and swallowing, and can be applied and removed with greater ease, as oppose to invasive ventilation. In patients with mild to moderate hypercapnic ARF secondary to chronic obstructive pulmonary disease (COPD) exacerbation, NIV can avert the need for intubation and invasive ventilation [6, 8, 9]. In hypercapnic patients, NIV can be used as an alternative to invasive ventilation [10, 11] and to favour the weaning process [12–15].
Fewer data are available on NIV for hypoxemic patients [16–18]. NIV has been used to prevent intubation in immunosuppressed patients with hypoxemic ARF [16–18] and as an alternative to invasive mechanical ventilation (i-MV) by a randomized controlled trial (RCT) including patients with acute lung injury (ALI) or acute respiratory distress syndrome (ARDS) [16]. No data are available, so far, on the use of NIV as a mean to facilitate the process of liberation from i-MV, in non-hypercapnic hypoxemic ARF patients. We designed this pilot randomized study to assess the feasibility of using NIV to wean patients with resolving hypoxemic ARF from i-MV.
Materials and methods
This pilot randomized controlled trial was performed at the Intensive Care Unit (ICU) of the University Hospital “Maggiore della Carità” in Novara (Italy), between March 2008 and March 2009, according to the principles outlined in the Declaration of Helsinki. The protocol was approved by the local ethics committee, and written informed consent was obtained for all patients. The trial was registered at the Australian New Zealand Clinical Trials Registry (Trial Number 083894).
Patients
We planned to enrol 20 consecutive patients. We considered eligible all intubated patients who met the following inclusion criteria: (1) age ≥18 years; (2) i-MV for at least 48 h; (3) pressure support ventilation (PSV) with a total applied pressure, i.e. positive end-expiratory pressure (PEEP) + inspiratory support, ≤25 cmH2O and a PEEP level between 8 and 13 cmH2O; (4) PaO2/FiO2 between 200 and 300 mmHg with a FiO2 ≤0.6; (5) PaCO2 ≤50 mmHg and pH ≥7.35; (6) respiratory rate (RR) ≤30/min; (7) core temperature <38.5 °C; (8) Glasgow coma scale (GCS) = 11; (9) cough on suctioning and need for tracheobronchial suctioning <2 per hour. Patients were excluded when they met any of the following exclusion criteria: (1) hemodynamic instability i.e. systolic arterial pressure <90 mmHg despite fluid repletion; (2) use of vasoactive agents, i.e. vasopressin, epinephrine and norepinephrine at any dosage, and dopamine or dobutamine >5 μg/kg/min; (3) life-threatening arrhythmias or electrocardiographic signs of ischemia; (4) severe sepsis [19]; (5) ARF secondary to neurological disorders, status asthmaticus, COPD, cardiogenic pulmonary oedema; (6) presence of tracheotomy; (7) uncontrolled vomiting; (8) two or more organ failures [20]; (9) body mass index ≥30; (10) documented history or suspicion of obstructive sleep apnoea; (11) inclusion in other research protocols. All ICU patients underwent a daily screening for study recruitment during the morning round. After enrolment, patients were allocated to either conventional invasive PSV (i-PSV) or noninvasive PSV (n-PSV) following a previously generated random sequence held by an investigator not involved in the study enrolment, who indicated in sealed, opaque numbered envelops the group of assignment. While the helmet was the first-choice interface for all 10 patients in the n-PSV group, full-face and oronasal masks were also utilized in rotation, to improve patient tolerance to NIV, as indicated.
Protocol
The study was performed using ICU ventilators with dedicated software for NIV application in PSV mode i.e. Servo-I (Maquet, Solna, Sweden) and EVITA 4 (Drager, Corsico, Italy). The ventilator was set with the same PEEP and inspiratory pressure support (PS) level applied during i-MV, setting the fastest pressure rise time. Heated humidification (HC 200, Covidien, Mansfield, MA) was used during i-MV, but not during NIV to avoid the fog effect consequent to accumulation of water in the helmet [21]. Heat and moisture exchange filters were used during NIV delivered by mask. The n-PSV patients could be sedated i.v. with either remifentanil [22, 23] (0.025–0.1 μg/kg/min) and/or propofol [22] (50 mg/h) to increase NIV tolerance. A similar sedation regimen was utilized for patients in the i-PSV group, but higher doses of remifentanil and propofol were allowed, as necessary. NIV was maintained continuously and briefly discontinued for no longer than 2–3 min, only when strictly necessary for changing the NIV interface. Patients maintained the semi-recumbent position. Enteral feeding via a nasogastric tube was administered according to the treating physician’s prescription, usually through a continuous infusion during 20–24 h. Gastric residual was checked every 4 h; when it exceeded 250 ml, promotility agents were added in therapy; when it exceeded 500 ml enteral feeding was discontinued and patient tolerance re-assessed [24].
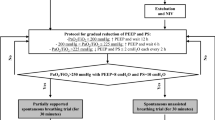
In the n-PSV group, PEEP and PS were decreased by 2 cmH2O each every 2 h till a minimum of 8 and 10 cmH2O, respectively, as shown in Fig. 1. During the weaning protocol the goal was to maintain PaO2/FiO2 ≥225 and PaCO2 ≤50 mmHg with a pH ≥7.35. In the case PaO2/FiO2 was less than 225 mmHg and at least 200 mmHg, PEEP was increased to reach the target of 225 mmHg and left at that level for 6 h before reattempting the reduction. When PaO2/FiO2 was less than 200 mmHg, PEEP was increased to reach the target, and left unchanged for 12 h. When PaO2/FiO2 exceeded 250 mmHg with PEEP 8 cmH2O and PS 10 cmH2O, a 30-min spontaneous breathing trial (SBT) was attempted with oxygen supplementation through a Venturi mask at a FiO2 of 0.35. NIV was interrupted if at the end of the SBT all the following occurred: pH ≥7.35, PaCO2 ≤50 mmHg and PaO2 ≥70 mmHg, RR ≤30 breaths/min, absence of dyspnoea, respiratory accessory muscles recruitment, and paradoxical abdominal motion.
Protocol flow chart. ETI endotracheal intubation, GCS Glasgow coma scale, i-MV invasive mechanical ventilation, PaO 2 /FiO 2 arterial oxygen tension to inspiratory oxygen fraction, i-PSV invasive pressure support ventilation, n-PSV noninvasive pressure support ventilation, PEEP positive end-expiratory pressure, PS pressure support, SB spontaneous breathing, SBT spontaneous breathing trial
Predefined criteria for re-intubation were cardiac or respiratory arrest; inability to protect the airway; coma or psychomotor agitation not controlled by continuous i.v. sedative infusion, as previously described [22, 23, 25]; unmanageable secretions or uncontrolled vomiting; life-threatening arrhythmias or electrocardiographic signs of ischemia; hemodynamic instability, as already described; intolerance to all interfaces; and two of the following: severe dyspnoea, PaO2/FiO2 <200 mmHg, and respiratory acidosis (pH <7.30 and PaCO2 >50 mmHg). Tracheotomy was performed after 14 days of i-MV when the treating physician considered prompt extubation unlikely or when the patient could not be extubated because of the inability to clear and remove secretions [12].
In the i-PSV group, PEEP and PS were titrated as already described for n-PSV. At the minimum level of 8 and 10 cmH2O of PEEP and PS, respectively, an SBT was conducted if PaO2/FiO2 was greater than 250 mmHg. The SBT consisted of 30 min of spontaneous breathing through the endotracheal tube with PEEP 5 cmH2O and PS 5 cmH2O. Patients were extubated if RR ≤30 breaths/min, pH ≥7.35, PaCO2 ≤50 mmHg and PaO2 ≥70 mmHg with a FiO2 of 0.35, without dyspnoea, respiratory accessory muscles recruitment, and paradoxical abdominal motion. The criteria for re-intubation and tracheotomy were the same as those already described for the n-PSV group.
Extubation failure was defined by the inability to sustain spontaneous unassisted breathing for 48 consecutive hours, without developing respiratory failure requiring ventilatory support (either invasive or noninvasive). The 48 h started for i-PSV at the time of extubation and for n-PSV after NIV was interrupted because the patient tolerated spontaneous unassisted breathing for 30 min.
In both groups, in those patients who failed because of dyspnoea with mild respiratory acidosis, i.e. 7.30 < pH < 7.35 and 45 mmHg < PaCO2 < 50 mmHg), without any other criteria of extubation failure, a “rescue” attempt of NIV was performed before intubation. Similarly, in the case of sole hypoxia (i.e. PaO2/FiO2 <200 mmHg) without other signs of respiratory failure, noninvasive continuous positive airway pressure (CPAP) was attempted.
Data analysis
Differences in arterial blood gases (ABGs) and RR between the two groups were assessed 1 h after enrolment (T1), and then after 12 (T12), 24 (T24) and 48 (T48) h. In addition, we evaluated and compared the rate of extubation failure (i.e. need for re-intubation or NIV application within 48 h after extubation), ICU and hospital mortality, time of weaning, duration of i-MV, number of septic complications, rate of tracheotomy, duration of continuous intravenous sedation and ICU length of stay.
Data are expressed as mean ± standard deviation (SD) or median ± interquartile range. The two groups were compared by using the Mann–Whitney test. Frequency distributions were compared by using chi-square test. A p value less than 0.05 was considered statistically significant.
Results
Clinical and functional characteristics of the two groups at enrolment are summarized in Table 1: age, gender, acute physiology and chronic health evaluation (APACHE) II score, PaO2/FiO2, and RR were similar in the two groups.
No patient required protocol discontinuation for intolerance in the treatment group. NIV was applied continuously for a median length of 37 h (13–72 h): six patients required continuous n-PSV for the first 24 h, four for 48 consecutive hours, four for 72 h, and two for 96 h, and one for more than 168 h. At extubation, the mean value of PEEP was 11 ± 1 and the mean value of PS was also 11 ± 1 cmH2O. Changes in ABGs between T0 (intubated) and the first hour after enrolment (T1) in i-PSV and n-PSV are shown in Table 2. No difference was found in PaO2/FiO2 and PaCO2. ABGs values were also similar between the two groups at T12, T24, and T48, as indicated in Table 2. One hour after the enrolment (T1), RR was significantly increased in n-PSV, but this difference vanished at the subsequent time points.
Only one patient failed discontinuation of mechanical ventilation within 48 h in the n-PSV group versus five patients in the i-PSV group (p = 0.051) (Table 3). In this group, two patients required re-intubation and were subsequently tracheotomized, the three other patients underwent “rescue” NIV for dyspnoea associate with mild respiratory acidosis. Of these, two were thereafter re-intubated, and one was subsequently tracheotomized. In the n-PSV group, the only patient who failed was immediately re-intubated because of dyspnoea and severe respiratory acidosis. Three patients in the i-PSV and one in the n-PSV group died in ICU (p = 0.58). Two of the three dead patients in the i-PSV group successfully completed the weaning protocol, but worsened within 48 h after extubation and developed multiple organ failure. The remaining patient died before extubation because of a comorbid condition i.e. severe cardiomyopathy. The patient who died in the n-PSV group developed pneumonia while on NIV and multiple organ failure after re-intubation. Regarding hospital mortality, one additional patient in the n-PSV group died after ICU discharge as a result of the comorbid conditions i.e. chronic kidney failure and diabetes.
Patients in the n-PSV group were free from i-MV for more days than those in the i-PSV group (1 ± 1 versus 9 ± 9 days of i-MV, p = 0.001) (Table 3). When the 28-i-MV-free days were taken into consideration this difference was still evident (10 ± 9 and 20 ± 8 days, for i-PSV and n-PSV, respectively, p = 0.014, min = 0, max = 25 days for both groups). The 28-MV-free days, i.e. invasive plus noninvasive MV, and the weaning time were similar between the groups (9 ± 9 versus 16 ± 9 days and 4 ± 6 versus 4 ± 4 days, for i-PSV and n-PSV, respectively). The rate of patients with septic complications was 50 % for i-PSV and 30 % for n-PSV (p = 0.36). Three patients were tracheotomized in the i-PSV group versus no patient in the n-PSV group (p = 0.06). The duration of continuous i.v. sedative infusion was not significantly different between the two groups (16 ± 11 in i-PSV versus 11 ± 8 days in n-PSV, p = 0.28). Table 4 reports the average dose of each drug and the number of patients requiring sedation at the different time points; though not statistically significant, there was a trend toward a reduced use of sedative drugs (i.e. lower doses and fewer patients) in n-PSV, as opposed to i-PSV. The ICU length of stay was 21 ± 13 days in the i-PSV group and 15 ± 11 days in n-PSV group (p = 0.28) (Table 3).
Discussion
Our pilot randomized controlled trial shows, in a small sample of highly selected patients, that early extubation followed by immediate application of NIV is feasible and might facilitate the liberation from mechanical ventilation in hypoxemic ARF patients. Available evidence supports the use of NIV as a weaning strategy for patients with acute on chronic respiratory failure [26], on the basis of RCTs including primarily patients with COPD exacerbation [12, 13, 15, 26]. In these studies, the duration of invasive mechanical ventilation [12, 13, 15], length of ICU and hospital stay [12, 15], septic complications [12, 15] and 90-day mortality [12] were all reduced in the treatment group, as opposed to controls. Very recently, Girault et al. [14] compared NIV with both conventional invasive ventilation and the oxygen therapy in weaning patients with hypercapnic ARF and found a significant reduction in the NIV group of the cumulative probability of post-extubation ARF, re-intubation or death. In the study by Ferrer et al. [12] 10 out of 43 patients were not hypercapnic, suggesting a potential role for NIV in facilitating weaning also in non-hypercapnic ARF.
This is the first study to investigate the feasibility of NIV to facilitate weaning in patients undergoing mechanical ventilation for hypoxemic ARF. Although the key factors determining the benefits of NIV in weaning patients with hypercapnic ARF may apply also to patients with h-ARF, the use of NIV in these patients is more difficult and complex [27]. All 10 patients included in the n-PSV group required at least 24 h of treatment; in six of them the time of NIV application was between 48 and 96 h. At enrolment, PEEP and PS were both on average 11 ± 1 cmH2O, to maintain a PaO2/FiO2 ratio of 240 ± 19 mmHg. Because continuous NIV application for prolonged periods of time and need for elevated airway pressures are both causes of NIV intolerance, reducing patient discomfort by using comfortable interfaces and cautiously administrating sedatives is extremely important in these patients.
To improve patient tolerance, we allowed a rotational use of three interfaces (helmet, full-face mask and oronasal mask), when needed. Three patients required all three interfaces, whereas in the remaining seven only the helmet was utilized. We chose the helmet as the first-choice interface because it is better tolerated, allowing the continuous application of NIV for prolonged periods of time [28, 29].
Low doses of i.v. sedatives were infused in five patients to improve NIV tolerance. In three patients we used only remifentanil, in one only propofol and in another one the two drugs were used in combination. Although one of the major benefits of NIV, as opposed to invasive ventilation, is avoiding heavy sedation, intolerance represents a major cause of NIV failure [11], which is associated with an increased risk of death [30]. The use of continuous i.v. sedative infusion during NIV aiming to achieve adequate patient comfort, while maintaining an acceptable patient cooperation, i.e. response to verbal stimulation, has been previously reported [22, 23, 31]. While Clouzeau et al. [31] administered propofol by target-controlled infusion (TCI), which makes a comparison with our data impossible, the dosage of remifentanil in our study (0.02 ± 0.01 μg/kg/min) was lower than those reported in previous studies by Rocco (0.07 ± 0.03 μg/kg/min) [23] and Constantin (0.10 ± 0.03 μg/kg/min) [22]. In all cases, in our study the rate of infusion allowed the patient to be calm, though fully cooperative on demand.
Our study has several limitations that deserve discussion. First, and most important, the number of patients included is small, which makes it impossible to draw conclusions on the real benefits of early extubation and NIV application in hypoxemic ARF patients. It is worth remarking that the intended aim of this clinical trial was just to assess the feasibility of NIV application after early extubation in hypoxemic patients. Considering the potential concerns on the use of NIV for weaning purposes in this specific patient population, which has never been systematically addressed by previous studies, we believe that a feasibility study was necessary for developing further properly powered clinical trials. Second, our study design makes blinding impossible, which might in principle introduce unintended biases. Third, because our trial was conducted in a single centre highly experienced with NIV and with the use of sedatives during NIV, our results might be not applicable to all ICUs. Finally, our patients were recovering from the episode of ARF and were accordingly rather stable, though dependent on relatively elevated airway pressures applied. The number of patients who failed extubation in the control group was as high as 50 %, which possibly depended on the clinical complexity of the patients enrolled; to our knowledge, however, no study has so far described the rate of extubation failure in this patient population. It is worth reminding that such an approach is likely unsafe in patients in the early dynamic phase of hypoxemic ARF.
Our data seem to exclude potential harm, as we could not detect differences in the rate of extubation failure, and ICU and hospital mortality, while suggesting potential benefits, as indicated by the fewer days spent on i-MV in the group of patients assigned to treatment, as opposed to controls. However, to ascertain whether or not early extubation and NIV application produce benefit in this patient population a properly powered multicentre trial including a much higher number of patients is clearly necessary.
Conclusions
Our pilot study shows that in highly selected patients with hypoxemic ARF, early extubation with immediate NIV application is feasible and probably not harmful. A properly powered multicentre randomized controlled trial may ascertain the real clinical benefits and definitely exclude the potential risks of this approach.
References
Esteban A, Anzueto A, Frutos F, Alia I, Brochard L, Stewart TE, Benito S, Epstein SK, Apezteguia C, Nightingale P, Arroliga AC, Tobin MJ (2002) Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA 287:345–355
Rello J, Diaz E, Roque M, Valles J (1999) Risk factors for developing pneumonia within 48 hours of intubation. Am J Respir Crit Care Med 159:1742–1746
Esperatti M, Ferrer M, Theessen A, Liapikou A, Valencia M, Saucedo LM, Zavala E, Welte T, Torres A (2010) Nosocomial pneumonia in the intensive care unit acquired by mechanically ventilated versus nonventilated patients. Am J Respir Crit Care Med 182:1533–1539
Bott J, Carroll MP, Conway JH, Keilty SE, Ward EM, Brown AM, Paul EA, Elliott MW, Godfrey RC, Wedzicha JA (1993) Randomised controlled trial of nasal ventilation in acute ventilatory failure due to chronic obstructive airways disease. Lancet 341:1555–1557
L’Her E, Deye N, Lellouche F, Taille S, Demoule A, Fraticelli A, Mancebo J, Brochard L (2005) Physiologic effects of noninvasive ventilation during acute lung injury. Am J Respir Crit Care Med 172:1112–1118
Plant PK, Owen JL, Elliott MW (2000) Early use of non-invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet 355:1931–1935
Appendini L, Patessio A, Zanaboni S, Carone M, Gukov B, Donner CF, Rossi A (1994) Physiologic effects of positive end-expiratory pressure and mask pressure support during exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 149:1069–1076
Brochard L, Mancebo J, Wysocki M, Lofaso F, Conti G, Rauss A, Simonneau G, Benito S, Gasparetto A, Lemaire F (1995) Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med 333:817–822
Kramer N, Meyer TJ, Meharg J, Cece RD, Hill NS (1995) Randomized, prospective trial of noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med 151:1799–1806
Conti G, Antonelli M, Navalesi P, Rocco M, Bufi M, Spadetta G, Meduri GU (2002) Noninvasive vs. conventional mechanical ventilation in patients with chronic obstructive pulmonary disease after failure of medical treatment in the ward: a randomized trial. Intensive Care Med 28:1701–1707
Squadrone E, Frigerio P, Fogliati C, Gregoretti C, Conti G, Antonelli M, Costa R, Baiardi P, Navalesi P (2004) Noninvasive vs invasive ventilation in COPD patients with severe acute respiratory failure deemed to require ventilatory assistance. Intensive Care Med 30:1303–1310
Ferrer M, Esquinas A, Arancibia F, Bauer TT, Gonzalez G, Carrillo A, Rodriguez-Roisin R, Torres A (2003) Noninvasive ventilation during persistent weaning failure: a randomized controlled trial. Am J Respir Crit Care Med 168:70–76
Girault C, Daudenthun I, Chevron V, Tamion F, Leroy J, Bonmarchand G (1999) Noninvasive ventilation as a systematic extubation and weaning technique in acute-on-chronic respiratory failure: a prospective, randomized controlled study. Am J Respir Crit Care Med 160:86–92
Girault C, Bubenheim M, Abroug F, Diehl JL, Elatrous S, Beuret P, Richecoeur J, L’Her E, Hilbert G, Capellier G, Rabbat A, Besbes M, Guerin C, Guiot P, Benichou J, Bonmarchand G (2011) Non-invasive ventilation and weaning in chronic hypercapnic respiratory failure patients: a randomized multicenter trial. Am J Respir Crit Care Med 184:672–679
Nava S, Ambrosino N, Clini E, Prato M, Orlando G, Vitacca M, Brigada P, Fracchia C, Rubini F (1998) Noninvasive mechanical ventilation in the weaning of patients with respiratory failure due to chronic obstructive pulmonary disease. A randomized, controlled trial. Ann Intern Med 128:721–728
Antonelli M, Conti G, Rocco M, Bufi M, De Blasi RA, Vivino G, Gasparetto A, Meduri GU (1998) A comparison of noninvasive positive-pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N Engl J Med 339:429–435
Antonelli M, Conti G, Bufi M, Costa MG, Lappa A, Rocco M, Gasparetto A, Meduri GU (2000) Noninvasive ventilation for treatment of acute respiratory failure in patients undergoing solid organ transplantation: a randomized trial. JAMA 283:235–241
Hilbert G, Gruson D, Vargas F, Valentino R, Gbikpi-Benissan G, Dupon M, Reiffers J, Cardinaud JP (2001) Noninvasive ventilation in immunosuppressed patients with pulmonary infiltrates, fever, and acute respiratory failure. N Engl J Med 344:481–487
Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, Calandra T, Dhainaut JF, Gerlach H, Harvey M, Marini JJ, Marshall J, Ranieri M, Ramsay G, Sevransky J, Thompson BT, Townsend S, Vender JS, Zimmerman JL, Vincent JL (2008) Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 36:296–327
ACCP/SCCM (1992) American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 20:864–874
Nava S, Navalesi P, Gregoretti C (2009) Interfaces and humidification for noninvasive mechanical ventilation. Respir Care 54:71–84
Constantin JM, Schneider E, Cayot-Constantin S, Guerin R, Bannier F, Futier E, Bazin JE (2007) Remifentanil-based sedation to treat noninvasive ventilation failure: a preliminary study. Intensive Care Med 33:82–87
Rocco M, Conti G, Alessandri E, Morelli A, Spadetta G, Laderchi A, Di SC, Francavilla S, Pietropaoli P (2010) Rescue treatment for noninvasive ventilation failure due to interface intolerance with remifentanil analgosedation: a pilot study. Intensive Care Med 36:2060–2065
Bankhead R, Boullata J, Brantley S, Corkins M, Guenter P, Krenitsky J, Lyman B, Metheny NA, Mueller C, Robbins S, Wessel J (2009) Enteral nutrition practice recommendations. JPEN J Parenter Enteral Nutr 33:122–167
Wong C, Burry L, Molino-Carmona S, Leo M, Tessler J, Hynes P, Mehta S (2004) Analgesic and sedative pharmacology in the intensive care unit. Dynamics 15:23–26
Burns KE, Adhikari NK, Keenan SP, Meade MO (2010) Noninvasive positive pressure ventilation as a weaning strategy for intubated adults with respiratory failure. Cochrane Database Syst Rev CD004127
Antonelli M, Conti G, Moro ML, Esquinas A, Gonzalez-Diaz G, Confalonieri M, Pelaia P, Principi T, Gregoretti C, Beltrame F, Pennisi MA, Arcangeli A, Proietti R, Passariello M, Meduri GU (2001) Predictors of failure of noninvasive positive pressure ventilation in patients with acute hypoxemic respiratory failure: a multi-center study. Intensive Care Med 27:1718–1728
Antonelli M, Pennisi MA, Pelosi P, Gregoretti C, Squadrone V, Rocco M, Cecchini L, Chiumello D, Severgnini P, Proietti R, Navalesi P, Conti G (2004) Noninvasive positive pressure ventilation using a helmet in patients with acute exacerbation of chronic obstructive pulmonary disease: a feasibility study. Anesthesiology 100:16–24
Antonelli M, Conti G, Pelosi P, Gregoretti C, Pennisi MA, Costa R, Severgnini P, Chiaranda M, Proietti R (2002) New treatment of acute hypoxemic respiratory failure: noninvasive pressure support ventilation delivered by helmet–a pilot controlled trial. Crit Care Med 30:602–608
Rocco M, Dell’Utri D, Morelli A, Spadetta G, Conti G, Antonelli M, Pietropaoli P (2004) Noninvasive ventilation by helmet or face mask in immunocompromised patients: a case-control study. Chest 126:1508–1515
Clouzeau B, Bui HN, Vargas F, Grenouillet-Delacre M, Guilhon E, Gruson D, Hilbert G (2010) Target-controlled infusion of propofol for sedation in patients with non-invasive ventilation failure due to low tolerance: a preliminary study. Intensive Care Med 36:1675–1680
Acknowledgments
Rosanna Vaschetto was supported by the ESICM Clinical Research Award 2009.
Conflicts of interest
P. Navalesi is working on the development of a new interface, not used in the present study, whose licence for patent belongs to Starmed S.P.A. If the new interface will be marketed, P. Navalesi will have some economic income. P. Navalesi has received honoraria/speaking fees from Maquet, Covidien, and GSK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vaschetto, R., Turucz, E., Dellapiazza, F. et al. Noninvasive ventilation after early extubation in patients recovering from hypoxemic acute respiratory failure: a single-centre feasibility study. Intensive Care Med 38, 1599–1606 (2012). https://doi.org/10.1007/s00134-012-2652-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2652-7