Abstract
Aims/hypothesis
In type 2 diabetic patients we compared 9 months of combination therapy with insulin glargine and metformin with 9 months of NPH insulin combined with metformin. The primary focus was changes in HbA1c; secondary focus was diurnal glucose profiles and symptomatic hypoglycaemia.
Methods
In this investigator-initiated open, parallel-group clinical trial involving seven centres, 110 insulin-naive type 2 diabetic patients with poor glycaemic control (HbA1c ≥8.0%) on oral hypoglycaemic agents (90% using sulfonylurea plus metformin) were randomised to receive bedtime insulin glargine with metformin (G+MET) or bedtime NPH with metformin (NPH+MET) for 36 weeks. The patients were taught how to self-adjust their insulin dose and use a modem to send the results of home glucose monitoring to treatment centres. The goal was to achieve a fasting plasma glucose (FPG) of 4.0 to 5.5 mmol/l in both groups.
Results
During the last 12 weeks, FPGs averaged 5.75±0.02 and 5.96±0.03 mmol/l (p<0.001) and insulin doses were 68±5 and 70±6 IU/day (0.69±0.05 and 0.66±0.04 IU kg–1 day–1, NS) in the G+MET and NPH+MET groups, respectively. At 36 weeks, mean HbA1c was 7.14±0.12 and 7.16±0.14%, respectively (NS). Symptomatic, but not confirmed symptomatic, hypoglycaemia was significantly lower during the first 12 weeks in the G+MET group (4.1±0.8 episodes/patient-year) than in the NPH+MET group (9.0±2.3 episodes/patient-year, p<0.05), but not significantly different thereafter. Glucose levels before dinner were higher in the NPH+MET group (10.1±0.3 mmol/l) than in the G+MET group (8.6±0.3 mmol/l, p=0.002) throughout the 36-week study. With regard to baseline characteristics such as initial glycaemia or C-peptide, there was no difference between patients who achieved good glycaemic control (HbA1c <7.0%) and those who did not. Differences were seen in the following: between study centres, weight gain during the run-in period and insulin therapy, and FPG during the last 12 weeks (5.7±0.2 vs 6.7±0.3 mmol/l for patients reaching vs those not reaching target, p<0.01).
Conclusions/interpretation
Good glycaemic control can be achieved with both G+MET and NPH+MET. Use of G+MET reduces symptomatic hypoglycaemia during the first 12 weeks and dinnertime hyperglycaemia compared with NPH+MET.
Similar content being viewed by others
Introduction
Meta-analyses [1, 2] and studies performed after these analyses [3, 4], such as the largest individual study, which was a substudy of the UK Prospective Diabetes Study [3], support the use of simple insulin combination therapy regimens for patients with type 2 diabetes. Regarding the choice of the insulin combination regimen, we previously compared four different bedtime NPH regimens, where NPH was combined with either a sulfonylurea, metformin, both, or another injection of NPH [5]. We found that use of metformin attenuated weight gain and its use was associated with less hypoglycaemia than the other combinations. Glycaemic control was also best with the NPH and metformin regimen, during which HbA1c decreased from 9.9 to 7.2% [5]. Similar data have been reported by others [6, 7].
Insulin glargine is a basal insulin analogue with a longer duration of action and smoother time–action profile than NPH [8]. Studies comparing sulfonylurea or sulfonylurea and metformin combined with insulin glargine have consistently found similar overall glycaemic control, similar insulin doses, but less hypoglycaemia than with NPH [4, 9, 10]. No study has, as yet, compared metformin monotherapy plus glargine with metformin monotherapy plus NPH. Given that use of metformin, either when combined with insulin [5] or when used without insulin [11], is associated with less hypoglycaemia than sulfonylurea, it is possible that the combination of metformin and glargine may not produce a reduction in hypoglycaemia compared with metformin and NPH.
Although insulin combination therapy regimens have resulted in similar or better glycaemic control than the use of insulin alone [1, 2, 12], even in the best studies HbA1c has averaged 7.0%, implying that approximately half of the patients remained inadequately controlled [3, 4]. There are no data explaining why some patients fail to achieve good glycaemic control during insulin combination therapy. Hypothetically, such failure could be the fault of the health-care professionals (e.g. insufficient advice on titration of insulin doses) or of the patients (e.g. poor adherence, fear of hypoglycaemia, inadequate insulin secretory reserve or antibody formation). In the present study we compared the combination therapy insulin glargine plus metformin (G+MET) with NPH insulin plus metformin (NPH+MET).
Subjects and methods
Study design
This was a multicentre, open, randomised, parallel-group study that compared the efficacy and safety of bedtime glargine with NPH insulin in combination with metformin in insulin-naive type 2 diabetic patients who were poorly controlled (HbA1c 8.0% or higher) and on oral hypoglycaemic agents. The study consisted of a 4-week run-in phase and a 36-week treatment phase. It was performed at six sites in Finland and one in the United Kingdom in accordance with the Declaration of Helsinki and good clinical practice as described by Note for Guidance CPMP/ICH/135/95. Approval by institutional ethics committees was obtained for each participating site. All patients provided written informed consent before entry to the study.
Patients, inclusion and exclusion criteria
Male or female patients aged 35–75 years with type 2 diabetes who had been treated with a stable dose (any dose) of sulfonylurea and metformin (≥1.5 g) or with metformin alone for at least 3 months prior to the screening visit at −4 weeks were enrolled. Further inclusion criteria included a BMI of 20–40 kg/m2, HbA1c ≥8.0%, a mean fasting plasma glucose (FPG) concentration of ≥7 mmol/l during daily home-glucose-monitoring between the screening visit at −4 weeks and the phone call in week −2, and fasting serum C-peptide ≥0.33 nmol/l (reference range 0.33–0.69 nmol/l). Exclusion criteria included use of other oral antihyperglycaemic agents, prior use of insulin, positive GAD antibodies or history of ketoacidosis, non-compliance with regard to daily measurement of FPG and one diurnal profile during the first 2 weeks of run-in (patients with <80% of all desired values were excluded), abnormal safety laboratory tests, including liver enzymes (serum alanine aminotransferase [S-ALT], serum aspartate aminotransferase [S-AST], serum alkaline phosphatase [S-AFOS]) higher than three times the upper limit of normal and serum creatinine ≥120 μmol/l (≥1.4 mg/dl), current or past history of alcohol or drug abuse, night shift work, pregnancy, treatment with any investigational drug in the 2 months before study entry, use of drugs likely to interfere with glucose control, clinically relevant major systemic disease other than diabetes that would make implementation of study protocol or interpretation of the results difficult, and mental health condition rendering the subject unable to understand the nature, scope and possible consequences of the study. Patients with diabetic retinopathy requiring surgical (laser or other) treatment in the 3 months before or during the study were also excluded.
Study protocol and treatment
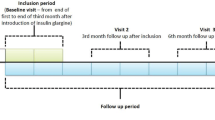
Screening visit at −4 weeks
At this visit, informed consent was obtained and the inclusion and exclusion criteria were reviewed. After a history had been taken and a physical examination performed, a fasting blood sample was taken for measurement of GAD antibodies, C-peptide, HbA1c, plasma glucose, blood counts, electrolytes, creatinine, C-reactive protein and liver enzymes. Other tests included ECG, urine analysis and a pregnancy test in women with child-bearing potential. The patients were taught home glucose monitoring and use of a modem to send glucose readings from home to the treatment centres (Fig. 1). No other education or lifestyle advice was recommended to be given at this or subsequent visits. The patients were asked to measure FPG every morning, and to perform a diurnal profile once during weeks −4 and −3 with plasma glucose measurements before and 2 h after breakfast, lunch and dinner, and at 22.00 and 04.00 h. Plasma glucose was measured using a glucose meter (Glucometer DEX 2; Bayer, Leverkusen, Germany). These data were used to assess compliance. During weeks −2 and −1 the patients were also asked to perform a diurnal profile once a week and to measure FPG daily. During the 36-week study, the patients were asked to perform a diurnal glucose profile at weeks 3, 5, 9, 13, 17, 23, 27, 31 and 35, i.e. on nine out of the 252 study days, and to measure FPG daily. All glucose data were sent by the patients via a modem to the treatment centre before scheduled phone calls. Adverse events including symptoms of hypoglycaemia were noted by the investigator at every visit and telephone contact.
Phone call at −2 weeks
This call was to verify compliance with glucose measurements and to review the results of the laboratory tests performed at −4 weeks. Eligible patients were randomly assigned to one of two groups (Table 1) using minimisation of differences (calculated for the variables listed below) between the treatment groups [13]. The following variables (the relative weight of each variable is given in parentheses) were considered: age (1×); sex (0.5×); BMI (1.5×); HbA1c (1.5×); duration of diabetes (0.5×); fasting serum C-peptide (1.0×); use of diuretics or beta-blocking agents (0.25×) or angiotensin-converting enzyme inhibitors (0.25×); previous use of a sulfonylurea (2×). This method of randomisation ensures accurate matching of baseline variables but does not necessarily result in groups of equal size [13].
Initiation of insulin therapy (0-week visit) and follow-up visits at 6, 12, 24 and 36 weeks
At the 0-week visit, sulfonylurea was discontinued and metformin continued unchanged. A similar educational programme was used in all participating centres. Insulin therapy was started if FPG still exceeded 7 mmol/l (126 mg/dl). The patients were taught to inject either glargine (Lantus; Aventis Pharma, Frankfurt, Germany) or NPH (Insuman Basal, Aventis Pharma) insulin using the Optipen Pro 1 pen (Aventis Pharma). The initial bedtime insulin dose was 10 IU for all patients who were using metformin alone, and 20 IU if the patients had used both sulfonylurea and metformin and sulfonylurea was stopped as was mandated by the study design. The goal was to achieve an FPG of 4.0–5.5 mmol/l (72–100 mg/dl) in both groups. The patients were taught to increase their insulin dose by 2 IU if FPG >5.5 mmol/l (100 mg/dl), and by 4 IU if FPG >10 mmol/l (180 mg/dl) on three consecutive mornings. Before the start of insulin therapy, and at weeks 12, 24 and 36, blood was withdrawn for measurements of full blood counts, electrolytes, creatinine, liver enzymes and lipids (cholesterol, triglycerides, HDL-cholesterol). At week 6, the patients visited the treatment centre to report adverse events and check their glucose meter. The correlation between FPG measured in the laboratory (7.9±0.2 mmol/l) and with the glucose meter (8.2±0.3 mmol/l) from the same blood sample was 0.91 (p<0.001). Body weight and blood pressure were recorded at each visit. In addition to sending glucose values via the modem, patients were asked to record their FPG and diurnal glucose measurements, as well as symptoms of hypoglycaemia, in a diary.
Phone calls at weeks −2, 1, 2, 4, 8, 10, 14, 16, 18, 20, 26, 28, 30, 32 and 34 weeks
The main purpose of the telephone calls was to encourage home glucose monitoring and self-adjustment of the insulin dose, and to record symptoms of hypoglycaemia. At the time of the phone call, the nurse in the treatment centre accessed the internet-site containing the patient’s glucose measurements (Fig. 1).
Analytical procedures
HbA1c was measured by high pressure liquid chromatography using the fully automated Glycosylated Hemoglobin Analyzer System (Bio-Rad, Richmond, CA, USA) traceable to the Diabetes Control and Complications Trial reference method, with a reference range of 4.0–6.0%. Serum concentrations of GAD-antibodies [14] and C-peptide [15] were determined by RIA. S-ALT, S-AST, S-AFOS activities were determined according to the recommendations of the European Committee for Clinical Laboratory Standards. Blood counts, creatinine concentrations and other laboratory measurements were performed using routine methods in local laboratories.
Efficacy and safety measures, sample size and statistical analyses
The primary efficacy measure was the change in HbA1c from baseline to endpoint. Secondary efficacy measures were diurnal glucose concentrations and symptomatic hypoglycaemia. Subjects were asked to self-monitor glucose values whenever they experienced symptoms that they thought might be the result of hypoglycaemia. Hypoglycaemic symptoms were recorded during phone calls. Biochemical hypoglycaemia was defined as a plasma glucose ≤4 mmol/l. Severe hypoglycaemia was defined as an event with symptoms consistent with hypoglycaemia during which the subject required the assistance of another person and which was associated with either a plasma glucose level <3.1 mmol/l or with prompt recovery after oral carbohydrate, i.v. glucose or glucagon administration. The sample size calculation was based on differences observed in a previous study between 11 insulin-naive patients treated with NPH and metformin and 12 patients treated with glargine and metformin for 1 year in Helsinki (a centre in the HOE901/3002 study) [9]. In this study HbA1c differed by 0.5% at the end of 1 year; the SDs for the groups were not different and averaged 0.87. The HbA1c change for the NPH + metformin group was −0.8±0.2% (mean±SE, n=11), and for the glargine + metformin group it was −1.3±0.3% (n=12) at the end of 1 year. Assuming α=0.05 and 80% power, the required number of patients per group to observe a difference of 0.5% is 50. To allow for a 10% drop out rate, 110 patients were randomised. When searching for predictors of the insulin dose at 9 months, simple correlation coefficients between all baseline variables and the insulin dose at 9 months were calculated. Variables significantly related to the insulin dose which were free of collinearity were chosen for multiple linear regression analysis. Diurnal profiles, weight, S-ALT, triglycerides and insulin doses between the groups were compared using two-way ANOVA followed by Bonferroni tests. Changes over time were analysed using repeated measures ANOVA followed by pairwise contrasts corrected for multiple comparisons using Systat Version 10.0 (SPSS, Chicago, IL, USA).
All statistical analyses were performed on an intent-to-treat basis, defined as randomised patients who received at least one injection of insulin. Statistical testing was performed at a significance level of α=0.05.
Results
Patient characteristics
A total of 157 patients were screened: 110 patients were eligible for randomisation and comprised the intent-to-treat population. Reasons for screening failure included HbA1c too low (n=18), positive GAD antibodies (n=9), active proliferative retinopathy (n=1), non-compliance or study too demanding (n=4), pancreatogenic diabetes (n=1), creatinine too high (n=2), alcohol abuse (n=1), BMI 51 kg/m2, other disease (n=4), other (n=6). Baseline demographic and clinical characteristics were similar between the treatment groups (Table 1). After randomisation, two patients discontinued the study (one on G+MET because of pancreatic cancer, and one on NPH+MET because of a pulmonary tumour, which turned out to be benign).
Glycaemic control
The time-course of FPG measured using the home glucose monitoring device is depicted in Fig. 2. The patients performed over 90% of the maximal recommended fasting daily glucose measurements (92 and 93%, n=16,180 and n=13,106 for G+MET and NPH+MET groups, respectively). During the run-in period, and during weeks 1–12, 13–24 and 25–36, FPGs were similar between the groups and averaged 11.3±0.1 vs 11.0±0.1, 7.8±0.04 vs 7.5±0.04, 6.0±0.03 vs 6.1±0.03, and 5.7±0.02 vs 6.0±0.03 mmol/l in the G+MET vs NPH+MET groups, respectively. HbA1c decreased from 9.13±0.15% at 0 weeks to 7.14±0.12% in the G+MET and from 9.26±0.15 to 7.16±0.14% in the NPH+MET group, NS (Fig. 2).
The diurnal profiles taken on the 9 days chosen from the 252 study days showed consistently lower glucose values before dinner (8.6±0.3 vs 10.1±0.3 mmol/l, p=0.002, G+MET vs NPH+MET, 0–36 weeks) in the G+MET than in the NPH+MET group (Fig. 3). During the first 12 weeks (means of three profiles), plasma glucose at 04.00 h was significantly higher in the G+MET group than in the NPH+MET group. This coincided with less hypoglycaemia during the same time period (see below). The CVs of diurnal plasma glucose (means of all diurnal profiles) were 24.0±0.8 and 22.2±0.9% (p=0.16, NS) during the run-in period, and 29.7±0.5 and 30.4±0.5% (p=0.30, NS) during the study proper for the G+MET and NPH+MET groups, respectively. The CVs of FPG levels during the run-in period and the study were also similar (data not shown).
a–d Diurnal plasma glucose profiles during the run-in period (a) and at 0–12 weeks (b), 13–24 weeks (c) and 25–36 weeks (d) in the G+MET and NPH+MET groups. The p values compare glucose values between the groups at a given time point. Filled circles, G+MET; open circles, NPH+MET; BB, before breakfast; AB, after breakfast; BL, before lunch; AL, after lunch; BD, before dinner; AD, after dinner; 22, 22.00 h; 04, 04.00 h. The corresponding frequencies of symptomatic hypoglycaemia (as episodes/patient-year) in the G+MET and NPH+MET groups during the run-in period (e), between 0 and 12 weeks (f), 13 and 24 weeks (g), and 25 and 36 weeks (h). *p<0.05 for glargine vs NPH groups
Hypoglycaemia
The number of episodes of symptomatic hypoglycaemia per patient was significantly lower in the G+MET than the NPH+MET group during the first 12 weeks of insulin therapy but thereafter the frequencies became similar (Fig. 3). The frequency of confirmed symptomatic hypoglycaemia during the first 12 weeks was 2.4 vs 5.6 episodes per patient during the first 12 weeks (NS). During the entire study, the frequency of hypoglycaemia averaged 5.4 (5.0 confirmed symptomatic) and 8.0 (7.7) episodes/patient-year in the G+MET and NPH+MET groups (p=0.12 for symptomatic and NS for confirmed symptomatic hypoglycaemia). Of the confirmed symptomatic hypoglycaemia, 98 and 93% in the G+MET and NPH+MET groups were nocturnal. The percentages of patients experiencing hypoglycaemia during run-in, and weeks 0–12, 13–24 and 25–36, were 5 vs 4% (NS), 46 vs 47% (NS), 43 vs 59% (p=0.08, NS) and 54 vs 57% (NS), respectively. There were no differences in biochemical hypoglycaemia (data not shown) and there were no episodes of severe hypoglycaemia.
Insulin dose
Insulin doses at 9 months averaged 68±5 and 70±6 IU/day (0.69±0.05 and 0.66±0.04 IU kg–1day–1, NS) in the G+MET and NPH+MET groups, respectively (Fig. 4).
In simple linear regression analysis, the following parameters measured at 0 weeks before insulin therapy were significantly correlated with the insulin dose (IU/day) at 36 weeks: body weight (r=0.53, p<0.0001), BMI (r=0.48, p<0.0001), S-ALT (r=0.38, p<0.0001), C-peptide (r=0.46, p<0.0001), HbA1c (r=0.28, p<0.005) and FPG (r=0.33, p<0.001). In multiple linear regression analysis, baseline body weight, S-ALT and HbA1c predicted the insulin dose at 9 months as follows: insulin dose (IU/day)=(−161±30)+(1.12±0.12)×weight (kg)+(0.38±0.10)×S-ALT (IU/l)+(11.9±2.9)×HbA1c (%), r=0.67, p<0.0001. The slopes or regression lines for predictors of the insulin dose at 9 months did not differ significantly between NPH and glargine insulin (data not shown).
Body weight
Mean weight gain during the 36 weeks in which the patients were treated with G+MET was 2.6±0.6 kg compared with 3.5±0.7 kg in patients treated with NPH+MET (NS) (Fig. 4).
Lipids and liver enzymes
Serum triglycerides decreased maximally and similarly by 12 weeks and then reached a plateau. In the G+MET group, serum triglycerides decreased by 29% from 2.3±0.2 at 0 months to 1.6±0.1 mmol/l at 9 months (p<0.001) and in the NPH+MET group by 27% from 2.5±0.2 to 1.8±0.1 mmol/l (p<0.001). HDL-cholesterol increased significantly in both groups, by 5%, from 1.18±0.04 to 1.24±0.04 mmol/l (p<0.02), in the G+MET group and by 6%, from 1.18±0.04 to 1.25±0.04 mmol/l (p<0.02), in the NPH+MET group. LDL-cholesterol remained unchanged in both groups (2.8±0.1 vs 2.8±0.1 mmol/l for 0 vs 9 months in the G+MET group and 2.9±0.1 vs 2.9±0.1 mmol/l in the NPH+MET group).
The decrease in serum ALT was highly significant and was 22% in both the G+MET and NPH+MET groups (Fig. 4).
Adverse events
The incidence of adverse events was similar: 33 patients (54%) in the G+MET group and 24 patients (49%) in the NPH+MET group reported at least one adverse event. Most common were infections and musculoskeletal and gastrointestinal disorders with no differences between the groups. One patient in the G+MET group and four in the NPH group had a serious adverse event (G+MET, endometriosis; NPH+MET, anaphylactic reaction, atrial fibrillation and cardiac failure, gastroenteritis, and pulmonary emphysema). These adverse events were considered to be unrelated to the study medications. Withdrawals as a result of serious adverse events occurred in one patient treated with G+MET (pancreatic cancer) and one patient treated with NPH+MET (suspected pulmonary cancer).
Relationship between FPG and HbA1c
To determine what HbA1c result corresponded to the predefined target FPG of 5.5 mmol/l, we calculated the relationship between FPG measured daily for the last 12 weeks and HbA1c measured at week 36. The relationship was not different for glargine vs NPH (data not shown) and was as follows: HbA1c (%)=([4.78±0.49]+[0.40±0.082])×FPG (mmol/l). The FPG of 5.5 mmol/l corresponded to an HbA1c of 6.96%.
Characteristics of patients reaching and not reaching an HbA1c ≤7%
The median HbA1c at 36 weeks was 7.0%. At baseline, before the run-in period, the patients reaching this HbA1c did not differ from those not reaching it with regard to age (57±1 vs 57±1 years for HbA1c <7.0 vs ≥7.0%), sex (38 vs 35% women), HbA1c (9.5±0.1 vs 9.6±0.1%), FPG (12.9±0.3 vs 13.1±0.4 mmol/l), BMI (31.3±0.7 vs 32.0±0.8 kg/m2), fasting C-peptide (1.00±0.05 vs 0.98±0.06 nmol/l) or other baseline parameters (previous oral or other drugs, family history, duration of diabetes, other laboratory parameters, data not shown). The patients who reached an HbA1c ≤7% gained significantly less weight before starting insulin therapy (−0.7±0.2 vs 0.2±0.2 kg, p<0.01 for HbA1c ≤7 vs HbA1c >7%), and this trend continued during insulin therapy (0.8±0.6 vs 1.6±0.6 kg, 1.4±0.5 vs 3.0±0.5 kg and 2.0±0.6 vs 4.0±0.6 kg, respectively, for weight gain during 12, 24 and 36 weeks, p<0.05). FPG was also significantly lower in those reaching the HbA1c target than in those who did not (5.7±0.2 vs 6.7±0.3 mmol/l, p<0.01). Insulin doses were not significantly different (61±4 vs 76±7 IU/day, p=0.08, 0.62±0.03 vs 0.73±0.05 IU kg–1 day–1, p=0.08, respectively).
We also explored whether there were differences between centres in the treatment response. The numbers of the centres do not follow the same order as in the list of authors. Centre 4 only recruited three patients and is not included. At baseline, there were no differences between centres in sex, baseline body weight or BMI, S-ALT, C-peptide or FPG. The groups differed slightly with regard to age (centres 1 [n=18], 2 [n=15], 3 [n=28], 5 [n=16], 6 [n=21], 7 [n=9]: 53±2, 56±3, 55±22, 57±2, 62±1, 56±3 years, respectively, p<0.05 for centre 6 vs the others) and 0-week HbA1c (centres 1, 2, 3, 5, 6, 7: 8.4±0.2, 9.5±0.3, 9.3±0.3, 9.4±0.2, 9.3±0.3, 9.4±0.2, 9.5±0.3%, respectively, p<0.02 for centre 1 vs the others). At 36 weeks, HbA1c values averaged 6.8±0.1, 6.8±0.3, 7.2±0.2, 7.8±0.3, 7.0±0.2 and 7.6±0.3%, respectively (p<0.02 for centre effect after adjusting for baseline age and for HbA1c due to centres 5 and 7). FPG levels during the last 12 weeks (6.0±0.2, 5.2±0.1, 5.8±0.2, 6.7±0.3, 5.6±0.2, 5.6±0.2 mmol/l, respectively), weight gain during the study (1.4±0.9, 0.13±1.0, 4.4±0.8, 3.0±0.9, 2.8±1.0, 6.5±1.7 kg, respectively) and insulin doses (0.57±0.05, 0.58±0.06, 0.86±0.07, 0.54±0.09, 0.58±0.05, 0.97±0.15 IU/kg, respectively) were also significantly different between the centres (p<0.001 for FPG, weight gain and insulin doses after adjusting for age and baseline HbA1c).
Discussion
The G+MET and the NPH+MET groups achieved comparable HbA1c: 7.14 and 7.16% at 9 months. FPG over the last 12 weeks averaged 5.75 and 5.96 mmol/l, respectively. The treat-to-target study also used only bedtime basal insulin in addition to oral agents and is perhaps the most relevant trial to compare the present results with, because it is (together with the study of Wright et al. [3]) the only large study in which glycaemic targets were on average achieved. As reviewed elsewhere, this has not been true for most studies in type 2 diabetes [1, 2]. In the present study, the achieved mean FPG levels were lower than in the treat-to-target study, where FPG averaged 6.50 and 6.67 mmol/l [4]. Although we achieved lower fasting glucoses than in the treat-to-target study, our HbA1c values were slightly higher than in the latter study, where HbA1c averaged 6.96 and 6.97% in the glargine and NPH groups. The reason for this discrepancy is unclear because the methods used to determine both plasma referenced blood glucose and HbA1c should have been comparable. Potential explanations include the use of sulfonylurea and metformin in the majority of patients in the treat-to-target study but of only metformin in the present study [4]. The possibly greater stimulation of endogenous insulin secretion by sulfonylurea in the treat-to-target study would have contributed to the discrepancy. We had no upper limit for HbA1c, in contrast to the treat-to-target study where baseline HbA1c averaged 8.6 compared with 9.5% in our patient group.
The group using G+MET experienced less symptomatic hypoglycaemia (episodes per patient) during the first 12 weeks. As the data on hypoglycaemic episodes were collected over the phone, the timing of these episodes and the prevailing glucose concentrations remain unknown, although the diurnal profile data during weeks 0–12 would suggest that the difference in hypoglycaemia was the result of nocturnal hypoglycaemia as the 04.00 h glucose was significantly lower in the NPH than the glargine group during this time period (Fig. 3). The lower rate of symptomatic hypoglycaemia is in keeping with previous studies showing less hypoglycaemia with glargine than with NPH when insulin has been combined with glimepiride alone [10] or with sulfonylurea and metformin [4, 9]. The difference in symptomatic hypoglycaemia was most marked during the first 12 weeks and then gradually disappeared (Fig. 3). During the last 12 weeks, symptoms of hypoglycaemia were similar in both groups. It is possible that the rather rapid lowering of plasma glucose in the beginning of the study induced symptoms even if the glucose values were still above normal, as has been documented in previous acute insulin infusion studies where hypoglycaemic symptoms have occurred by lowering glucose from markedly hyperglycaemic to less hyperglycaemic concentrations [16]. The finding that symptoms of hypoglycaemia no longer differed at the end of the study may explain why hypoglycaemia did not become rate-limiting for titration of the insulin dose, and why average glycaemic control was similar between the groups.
The diurnal profile data resemble closely those previously reported in the study comparing addition of glargine or NPH to other oral agents, i.e. glucose levels around dinnertime were significantly lower with glargine than NPH [9]. These differences did not translate into differences in HbA1c either in the present study or in the earlier studies, possibly because glucose concentrations were higher in the glargine than the NPH group during the night. The reason why higher nocturnal glucose values were not consistently observed in the diurnal profiles is unclear but could be the result of a type 2 error, i.e. diurnal profiles were only measured on nine out of the 252 study days. It is also possible that the 04.00 h time for measurement may have missed the nocturnal glucose nadir.
Analysis of previous studies [12] and direct measurements of components of energy balance during insulin therapy [17] have shown that one may predict approximately 2 kg of weight gain for every 1% unit drop in HbA1c with insulin alone, or insulin and sulfonylurea combination therapy [12]. In the present study, weight gain averaged 1.3±0.4 kg/% decrease in HbA1c in the G+MET group and 2.2±0.9 kg/% in the NPH+MET group (NS), respectively. Although metformin has attenuated weight gain in previous studies during insulin therapy, i.e. the gain in body weight has been less than the 2 kg/1% decrease in HbA1c, in these studies the patients were metformin-naive [5] while in the present study the patients were using metformin when insulin therapy was started. Other factors may also influence weight gain. As discussed below, a novel finding in this study was the effect of study centre on weight gain, i.e. patients who achieved their target HbA1c gained less weight both during the run-in period and during insulin therapy than those who failed to achieve their glycaemic targets.
There are no previous data on predictors of insulin requirements in type 2 diabetes. Glucose uptake, because of the stimulating effect of hyperglycaemia per se, is normal or increased despite insulin resistance in type 2 diabetes [18, 19]. Therefore blood glucose can only be decreased by inhibiting hepatic glucose production unless oxygen consumption is increased [20, 21]. One may therefore expect that hepatic insulin resistance is an important determinant of the insulin dose independent of body mass. We have previously shown that hepatic fat content is significantly correlated with S-ALT [22] and hepatic insulin resistance [23, 24]. Similarly, in a small group of type 2 diabetic patients, we previously found liver fat measured with proton spectroscopy to correlate closely with insulin requirements and hepatic insulin resistance [23]. Not unexpectedly, another predictor of the total insulin dose (IU/day) was body weight. Baseline HbA1c was a third independent predictor of insulin requirements, possibly because poor glycaemic control implies advanced metabolic abnormalities. Altogether we could explain 45% of the variation in insulin dose from these baseline measurements. The predictive equation is not accurate enough to be used on an individual basis but helps in understanding the large inter-individual variation in insulin requirements.
It is commonly assumed that a patient who fails to achieve good glycaemic control with simple basal insulin combination therapy regimens needs more intensive insulin therapy, such as a basal–bolus regimen, possibly because of poor beta cell function and postprandial hyperglycaemia. So far this hypothesis remains unsupported by clinical trials. It is also often thought that it is the patient alone who is the sole reason for failure to achieve good glycaemic control. In the present study we found highly significant differences between the participating centres with respect to achieved fasting glucoses and HbA1c values, insulin doses and weight gain during insulin therapy. These differences could not be attributed to differences in the baseline characteristics of the patients. These data suggest that the teaching skills of the diabetes care team contribute to the success of insulin therapy.
To conclude, good glycaemic control can be achieved using a simple insulin initiation protocol and combination therapy with either glargine and metformin or NPH and metformin. Use of glargine was associated with fewer symptoms of hypoglycaemia during the first 12 weeks of insulin therapy and with better dinnertime glucose control throughout the study.
Abbreviations
- G+MET:
-
glargine + metformin
- FPG:
-
fasting plasma glucose
- NPH+MET:
-
NPH insulin + metformin
- S-AFOS:
-
serum alkaline phosphatase
- S-ALT:
-
serum alanine aminotransferase
- S-AST:
-
serum aspartate aminotransferase
References
Pugh JA, Wagner ML, Sawyer J, Ramirez G, Tuley M, Friedberg SJ (1992) Is combination sulfonylurea and insulin therapy useful in NIDDM patients? Diabetes Care 15:953–959
Peters AL, Davidson MB (1991) Insulin plus a sulfonylurea agent for treating type 2 diabetes. Ann Intern Med 115:45–53
Wright A, Burden AC, Paisey RB, Cull CA, Holman RR (2002) Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the U.K. Prospective Diabetes Study (UKPDS 57). Diabetes Care 25:330–336
Riddle MC, Rosenstock J, Gerich JE, Insulin glargine 4002 study investigators (2003) The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy in type 2 diabetic patients. Diabetes Care 26:3080–3086
Yki-Järvinen H, Ryysy L, Nikkilä K, Tulokas T, Vanamo R, Heikkilä M (1999) Comparison of bedtime insulin regimens in patients with type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med 130:389–396
Aviles-Santa L, Sinding J, Raskin P (1999) Effects of metformin in patients with poorly controlled, insulin-treated type 2 diabetes mellitus. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 131:182–188
Wulffele MG, Kooy A, Lehert P et al (2002) Combination of insulin and metformin in the treatment of type 2 diabetes. Diabetes Care 25:2133–2140
Lepore M, Pampanelli S, Fanelli C et al (2000) Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes 49:2142–2148
Yki-Jarvinen H, Dressler A, Ziemen M (2000) Less nocturnal glycaemia/hypoglycaemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care 23:1130–1136
Fritsche A, Schweitzer MA, Haring HU (2003) Glimepiride combined with morning insulin glargine, bedtime neutral protamine hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes. A randomized, controlled trial. Ann Intern Med 138:952–959
DeFronzo RA, Goodman AM (1995) Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicentre Metformin Study Group. N Engl J Med 333:541–549
Yki-Järvinen H (2001) Combination therapies with insulin in type 2 diabetes. Diabetes Care 24:758–767
Taves DR (1974) Minimization: a new method of assessing patients to treatment and control groups. Clin Pharmacol Ther 15:443–453
Tuomi TM, Groop LC, Zimmet PZ, Rowley MJ, Knowles W, Mackay IR (1993) Antibodies to glutamic acid decarboxylase reveal latent autoimmune diabetes mellitus in adults with non-insulin-dependent onset of disease. Diabetes 42:359–362
Kuzuya H, Blix PM, Horwitz DL, Steiner DF, Rubenstein AH (1977) Determination of free and total insulin and C-peptide in insulin-treated diabetics. Diabetes 26:22–29
DeFronzo RA, Hendler R, Christensen N (1980) Stimulation of counterregulatory hormonal response in diabetic man by a fall in glucose concentration. Diabetes 29:125–131
Mäkimattila S, Nikkilä K, Yki-Järvinen H (1999) Causes of weight gain during insulin therapy with and without metformin in patients with non-insulin-dependent diabetes mellitus. Diabetologia 42:406–412
Yki-Järvinen H, Helve E, Sane T, Nurjhan N, Taskinen M-R (1989) Insulin inhibition of overnight glucose production and gluconeogenesis from lactate in NIDDM. Am J Physiol 256:E732–E739
Gerich JE, Mitrakou A, Kelley D et al (1990) Contribution of impaired muscle glucose clearance to reduced postabsorptive systemic glucose clearance in NIDDM. Diabetes 39:211–216
Mitrakou A, Kelley D, Veneman T et al (1990) Contribution of abnormal muscle and liver metabolism to postprandial hyperglycaemia in NIDDM. Diabetes 39:1381–1390
Pehling G, Tessari P, Gerich JE, Haymond MW, Service FJ, Rizza RA (1984) Abnormal meal carbohydrate disposition in insulin-dependent diabetes. Relative contributions of endogenous glucose production and initial splanchnic uptake and effect of intensive insulin therapy. J Clin Invest 74:985–991
Westerbacka J, Corner A, Tiikkainen M et al (2004) Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: implications for sex differences in markers of cardiovascular risk. Diabetologia 47:1360–1369
Ryysy L, Häkkinen AM, Goto T et al (2000) Hepatic fat content and insulin action on free fatty acids and glucose metabolism rather than insulin absorption are associated with insulin requirements during insulin therapy in type 2 diabetic patients. Diabetes 49:749–758
Seppala-Lindroos A, Vehkavaara S, Hakkinen AM et al (2002) Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab 87:3023–3028
Acknowledgements
Data from this manuscript have been published as abstracts at the American Diabetes Association meeting in Orlando 2004 and at the European Association for the Study of Diabetes meeting in Munich 2004. This investigator-initiated study was supported by grants from the Academy of Finland and from Aventis (Bridgewater, NJ, USA).
Duality of interest H. Yki-Järvinen has acted as a consultant or speaker for Amylin, Astra-Zeneca, Aventis, Lilly and MSD and received grant support for investigator-initiated trials from Aventis, Lilly, Novartis and Roche.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yki-Järvinen, H., Kauppinen-Mäkelin, R., Tiikkainen, M. et al. Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetologia 49, 442–451 (2006). https://doi.org/10.1007/s00125-005-0132-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-005-0132-0