Abstract
Background: Sleep disturbances and their potential association with stroke remains understudied at a population level. We sought to determine the prevalence of sleep disturbances among people who have effects of stroke compared with the general population.
Methods: We used data from people aged 18 years or older who responded to the sleep and chronic disease modules of the 2017–2018 cycle of the Canadian Community Health Survey (CCHS). We measured sleep disturbances by self-reports of having trouble staying awake most or all of the time; either short (< 5 h) or long (> 9 h) nightly sleep duration; having trouble going to or staying asleep most or all of the time; and never, rarely or sometimes having refreshing sleep. We used log-binomial and multinomial regression to investigate prevalence of sleep disturbances among respondents who reported effects of stroke compared with others, adjusting for confounding factors.
Results: We included 46 404 CCHS respondents, 682 of whom reported effects of stroke. The prevalence of sleep disturbances for those with effects of stroke was higher than among others in the sample with regard to trouble staying awake (13.0% v. 6.1%; adjusted relative risk [RR] 2.16, 95% confidence interval [CI] 1.59–2.94), short or long duration sleep (28.9% v. 10.0%; adjusted RR 1.93, 95% CI 1.57–2.38), trouble going to or staying asleep, (28.1% v. 17.6%; adjusted RR 1.53, 95% CI 1.28–1.83) and lack of refreshing sleep (41.1% v. 37.1%; adjusted RR 1.30, 95% CI 1.14–1.49). The prevalence of at least 1 reported measure of sleep disturbance was 61.6% among those with effects of stroke, compared with 48.2% among others (adjusted RR 1.28, 95% CI 1.18–1.40).
Interpretation: Self-report of having effects of stroke was associated with increased prevalence of sleep disturbances compared with the general population. Sleep disturbances were reported by a high proportion of respondents with effects of stroke, indicating the importance of screening for related disorders.
Disturbance of normal sleeping patterns is a commonly reported, but understudied, condition among people who have had a stroke.1 Sleep disturbances represent both a risk factor for and a consequence of stroke.2–8 Sleep disturbances are thought to have a negative impact on functional recovery of activities of daily living, mood and levels of fatigue.9,10 When interviewed, people who have had a stroke reported both reduced and excessive sleep afterward.11 International consensus exists that cognitive and other nonmotor impairments are priority areas for stroke research.12,13 Furthermore, understanding poststroke fatigue (and how to reduce it), to which sleep disruptions may be a major contributor,14 is one of the top 10 patient priorities for stroke rehabilitation.15
Despite the known association between sleep disturbances and stroke, the prevalence of sleep disturbances among patients with stroke is not well defined. Studies of the prevalence of sleep disturbances are often limited by small sample size, providing estimates that range between 25%16 and 80%,17 with a wide variety of stroke characteristics and populations included across studies. Meta-analyses have estimated a prevalence of poststroke insomnia of 29%–54%, depending on the length of time after stroke, but this neglects those who have poststroke hypersomnia.6
Our aim was to determine the prevalence of sleep disturbances among people who have or have not had stroke; we hypothesized that the prevalence of sleep disturbances is higher among those who have effects of a stroke compared with the general population.
Methods
Study design
We conducted a cross-sectional, population-based study using data from adults who completed the 2017–2018 Canadian Community Health Survey (CCHS) including the module on sleep disturbances.
Data source and study population
The CCHS is a multistage, stratified cluster survey with data that represent 98% of the Canadian population aged 12 years or older. The 2017–2018 survey cycle (the most recent cycle available for public use at the time of study conduct) had 113 290 respondents.18 We restricted analysis to adults (age ≥ 18 yr) living in Prince Edward Island, Quebec, Alberta, British Columbia, Yukon Territory and Nunavut, where the relevant sleep-related question module was administered. We excluded those with missing data on the primary exposure of interest (stroke status) or on any of the potential covariates.
Outcomes
We measured 4 types of sleep disturbances according to the following responses to questions in the CCHS: most or all of the time to, “How often do you find it difficult to stay awake when you want to?”; short (< 5 h) or long (> 9 h) self-reported sleep durations each night to, “How long do you usually spend sleeping each night?”; most or all of the time to, “How often do you have trouble going to sleep or staying asleep?”; and never, rarely or sometimes to, “How often do you find your sleep refreshing?”. The full set of potential responses to scale questions were “never,” “rarely,” “sometimes,” “most of the time” and “all of the time.” The specific composition of the sleep-related question set in the CCHS has not been validated as a diagnostic tool; however, similar questions and 5-point scales are used as components of other sleep disorder questionnaires.19–22 We selected thresholds to be consistent with methods used in other studies that have used the CCHS sleep module.23–26 Previous studies using CCHS data have shown the association of both short and long sleep duration with chronic disease, which informed our rationale for selecting these cutoffs.2,24
Exposure
We determined stroke status from the CCHS question, “Do you suffer from the effects of a stroke?” This question has been shown to have moderate agreement (κ = 0.58) with physician claims data indicating presence of stroke.27 Hereafter, we refer to respondents who said yes to this question as having had a stroke.
Confounders
We used previous CCHS analyses,2,23–25 primary studies in the clinical setting,10,11,14,16,17,28 meta-analyses,6,9,29 literature reviews8,30,31 and clinical expertise of the coauthors to select covariates with associations to both sleep disturbances and stroke. Model 1 used a set of confounders with a low likelihood of being colliders, namely diabetes status, age, sex, highest level of education, body mass index, physical activity level according to the Canadian Physical Activity Guidelines (moderate-to-vigorous activity ≥ 150 min/wk), frequency of alcohol use and frequency of smoking. Model 2 included all confounders in Model 1, with the addition of other self-reported chronic conditions, namely arthritis, asthma, cancer, heart disease, high blood cholesterol, high blood pressure, mood disorder (e.g., depression, bipolar disorder, mania, dysthymia) and anxiety disorder (e.g., phobia, obsessive–compulsive disorder, panic).4,14,25,29,32,33 These factors have potential bidirectional relationships with our exposure and outcomes. Depending on the temporality of these factors (which is unknown, given the cross-sectional design) they could act as either confounders or colliders, leading to a more complete set of covariates, but a potential risk of overadjustment in Model 2. We recoded the original CCHS age variable into 6 age categories (18–39 yr, 40–49 yr, 50–59 yr, 60–69 yr, 70–79 yr, ≥ 80 yr) to better represent age ranges by which prevalence of insomnia has been previously found to vary and to provide a sufficient sample size of people who have had a stroke within each category, as stroke is relatively uncommon at younger ages.23
Statistical analysis
We adjusted all point and variance estimates using relative weights (individual sampling weight divided by average weight of all included respondents) and the average design effect for Canada (2.95) provided in the CCHS documentation.18 We used these values to calculate an adjusted weight (relative weight divided by the square root of the design effect) that was incorporated into all analyses to account for the complex survey design of the CCHS. We tabulated frequencies using these adjusted weights to describe the prevalence of each type of sleep disturbance by stroke status and across covariates. We also calculated the risk of having at least 1 of the 4 sleep disturbances of interest. We used log-binomial regression to calculate relative risks (RRs) with 95% confidence intervals (CIs) of the association between sleep disturbances and stroke and covariates. For each type of sleep disturbance, we investigated sex and age as possible effect modifiers of stroke. We used multinomial logistic regression to compare the ratio of RRs of having increasing numbers of co-occurring sleep disturbances, compared with 0 sleep disturbances, by stroke status. We considered a p value of less than 0.05 to be statistically significant. We present prevalence data as absolute risks, and model outputs as RRs by stroke status. We have reported all results in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement and CMAJ guidance for survey reports.34,35 We performed all analyses using SAS version 9.4 (SAS Institute).
Ethics approval
Institutional ethics approval was not required for this study, as it solely used publicly accessible data sources.
Results
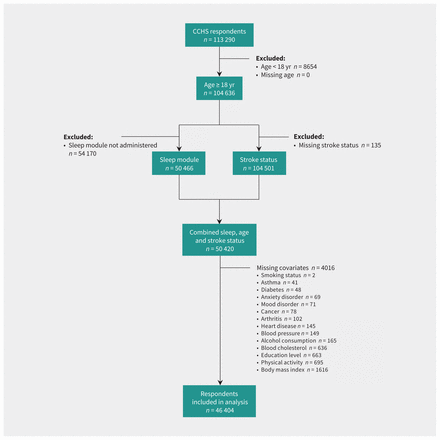
We included 46 404 respondents, 682 of whom reported that they suffered from the effects of a stroke (Figure 1). Differences between participants with and without stroke are provided in Table 1. After adjusting for survey weight and design effect, the prevalence of stroke was 1.1% and increased with age (18–39 yr: 0.2%; 40–49 yr: 0.3%; 50–59 yr: 1.1%; 60–69 yr: 2.1%; 70–79 yr: 2.8%; ≥ 80 yr: 5.4%). Overall, the prevalence of at least 1 type of sleep disturbance (61.6% v. 48.2%), as well as all 4 individual types of sleep disturbances (13.0%–41.1% v. 6.1%–37.1%) was higher among those who had a stroke than among others (Table 2). Prevalence for each type of sleep disturbance, stratified by stroke status and all modelled covariates, is available in Appendix 1, Sections 1–5, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.221063/tab-related-content.
Study flowchart. Note: CCHS = Canadian Community Health Survey.
Survey participant characteristics
Prevalence of self-reported sleep disturbances in Canadian adults by stroke status*
We did not observe any significant interactions of stroke with sex or age, but stratified RRs are provided in Section 6 and 7 of Appendix 1. Respondents who suffered from the effects of a stroke were more likely to report at least 1 type of sleep disturbance in the unadjusted model (RR 1.28, 95% CI 1.17–1.40) and in Model 1 (adjusted RR 1.28, 95% CI 1.18–1.40), but not in Model 2 (adjusted RR 1.06, 95% CI 0.98–1.15) (Table 3). This group also had an increased risk of difficulty staying awake (unadjusted RR 2.13, 95% CI 1.58–2.88; Model 1 adjusted RR 2.16, 95% CI 1.59–2.94; Model 2 adjusted RR 1.83, 95% CI 1.35–2.49), and of short or long sleep duration (unadjusted RR 2.88, 95% CI 2.40–3.47; Model 1 adjusted RR 1.93, 95% CI 1.57–2.38; Model 2 adjusted RR 1.50, 95% CI 1.22–1.85) (Appendix 1, Sections 8 and 9). People who reported a stroke had higher risk of difficulty going to sleep in both the unadjusted model (RR 1.60, 95% CI 1.33–1.92) and in Model 1 (adjusted RR 1.53, 95% CI 1.28–1.83), but this was attenuated and became nonsignificant when controlling for additional covariates in Model 2 (adjusted RR 1.11, 95% CI 0.91–1.35) (Appendix 1, Section 10). People who reported a stroke had a significantly higher risk of nonrefreshing sleep only in Model 1 (adjusted RR 1.30, 95% CI 1.14–1.49) (Appendix 1, Section 11). The effects of extreme sleep duration were similar when short and long durations were considered separately, so we favoured combining these categories (Appendix 1, Section 12).
Associations between having at least 1 type of self-reported sleep disturbance, self-reported stroke and other covariates
The proportion of respondents with 0, 1, 2, 3 or 4 types of sleep disturbances is provided in Table 4. The RR ratio of having either 3 or 4 types of sleep disturbances, compared with 0 disturbances, among respondents who reported a stroke was significantly higher than among those without stroke effects in all models (Table 4). The RR ratio for 2 co-occurring sleep disturbances was significantly higher for those who reported a stroke in the unadjusted model and in Model 1. The RR ratio for any 1 type of sleep disturbance was higher among people who reported a stroke only in Model 1 (adjusted RR 1.40, 95% CI 1.05–1.87).
Proportions and relative risk ratios of co-occurring sleep disturbances, compared with 0 sleep disturbances, by stroke status*
Interpretation
All 4 types of sleep disturbances were more prevalent among people who reported a stroke than in the general population. The most common type of sleep disturbance among those who reported a stroke was nonrefreshing sleep, whereas the type of sleep disturbance with the highest RR for this group was difficulty staying awake. This group had a significantly greater risk of having more co-occurring sleep disturbances than those who did not report a stroke, reinforcing the potential association between stroke status and each type of sleep disturbance. Overall, the interaction between stroke and sex or age was not significant, indicating that the association of sleep disturbances with stroke was similar for both sexes and across the lifespan.
We found that almost two-thirds of people who reported a stroke also had at least 1 type of sleep disturbance, with a risk of sleep disturbances 1.3–2.2 times higher than the general population, depending on the specific type of disturbance. The risk of having co-occurring sleep disturbances was 1.9–7.4 times higher. One CCHS study previously showed that people with excess sleep duration (≥ 10 hr) had a higher risk of stroke (adjusted hazard ratio 2.24, 95% CI 1.01–4.98) than those sleeping 7–9 hours per night, similar to our risk estimates for short or long sleep duration.2 Others have identified associations between sleep duration and difficulty going to sleep with various chronic diseases, but not stroke specifically.24,25 Our study advances this work by showing associations between all types of sleep disturbances measured in the CCHS, including both short and long sleep duration, as well as the increased risk of co-occurring sleep disturbances in those who have had a stroke. Other studies have shown that sleep disorders are highly prevalent among those who have had a stroke, and that prevalence decreases over time in this population, yet remains significantly elevated.6,36 Even in the chronic (> 6 mo)37 phase after stroke, sleep-disordered breathing and insomnia occur in 59%–72% and 32%–50% of people who have had a stroke, respectively.6,36 Although we did not measure diagnosed sleep disorders, we found that related sleep disturbances, which could be self-reported as part of a screening tool during routine visits,20 had a similar prevalence among people who reported a stroke.
Limitations
The cross-sectional nature of the CCHS prevents us from establishing whether reported sleep disturbances were present before stroke or developed after stroke. Symptoms related to sleep disturbances have been previously established as both risk factors for and consequences of stroke.3,17 Data were self-reported and not objectively measured, given the nature of the survey. Although self-reported and objective measures of sleep disturbances may not correlate in the acute phase after stroke, they are correlated at 6 month follow-up.38 However, we did not know the time between occurrence of stroke and survey completion.
Covariates adjusted in our models were limited by the data available within the CCHS questionnaire. Other potential confounders that may have important impacts on sleep disturbances and that are associated with stroke, such as sleep-disordered breathing, could be considered in future work.8,17,39 Some covariates may have created an overadjustment bias from collider effects in the cross-sectional data. Despite this, we showed the effect of stroke on several types of sleep disturbances using a model that may have residual confounding but low risk of overadjustment (Model 1) and another that was more comprehensively controlled, but with greater risk of overadjustment (Model 2).
The module of sleep-related questions was administered only in 6 Canadian regions (not including Ontario, the most populous province in Canada). As such, readers should be cautious in generalizing the results beyond the provinces in which data were collected. Our results do not represent a pan-Canadian estimate.
Finally, the overall prevalence of stroke that we observed in CCHS data (1.08%) was lower than that observed in Canadian Chronic Disease Surveillance System (CCDSS) data (2.92%).40 This lower prevalence of stroke in the CCHS was more pronounced with increasing age (e.g., 0.28% in CCHS v. 0.62% in CCDSS among people aged 35–49 yr, 5.41% in CCHS v. 17.07% in CCDSS among those aged ≥ 80 yr).40 Data from the CCHS are self-reported by voluntary participants, whereas diagnoses in the CCDSS are based on information from hospital admissions and physician billing data, which are also subject to misclassification bias.41–43 People who had severe strokes may have been less willing or able to participate in the survey than those with mild strokes or no strokes, which may have created a selection bias in our data. Individuals living in institutions are excluded from CCHS survey coverage,18 so those who had very severe strokes that resulted in institutionalization would not have been captured. Conversely, the CCHS question, “Do you suffer from the effects of a stroke?” may have caused some people without residual impairments, or those who did not feel they “suffered” from their stroke, to answer “no” to this question. It is difficult to estimate how this incomplete ascertainment may have biased our findings, as both those with the least and most severe levels of stroke impairment may be under-represented. Future studies could attempt to link CCHS data to other provincial health administrative data sets that include information on the time elapsed since stroke and stroke severity to explore associations with these factors, as well as a wider range of potential confounders and follow-up.
Conclusion
We estimated the prevalence and association of sleep disturbances with stroke, showing that almost two-thirds of people in several regions of Canada who reported having had a stroke also reported at least 1 type of sleep disturbance. The risk of having co-occurring sleep disturbances was also significantly elevated in this group. Sleep disturbances affect quality of life and are highly prevalent among people who have had a stroke. Family physicians and stroke specialists could consider screening for sleep disturbances during routine visits, as an addition to the ongoing care of this population. The adaptation of stroke-specific sleep interventions is an emerging area, and increased knowledge of the high prevalence of these disturbances will help physicians make the best care decisions for patients who have had a stroke.44
Acknowledgement
The authors acknowledge Dr. Marie-Hélène Roy-Gagnon for her assistance with the multinomial logistic regression analysis.
Footnotes
Competing interests: Tetyana Kendzerska is supported by the Physicians’ Services Incorporated Graham Farquharson Knowledge Translation Fellowship. She also received a speaker honorarium from Astra-Zeneca Canada and is a clinical consultant at Pitolisant Medical Advisory Board (Paladin Labs Inc.). Kathryn Hayward reports funding from the Medical Research Future Fund, outside the submitted work. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Matthew Jeffers, Alison Pittman, Dale Corbett, Kathryn Hayward and Yue Chen conceptualized and designed the study. Matthew Jeffers, Alison Pittman and Tetyana Kendzerska contributed to data collection, analysis and interpretation. Matthew Jeffers and Alison Pittman drafted the manuscript. All of the authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: No funding was received to directly support this study. Matthew Jeffers is supported by the Ontario Graduate Scholarship and a salary award from the BLUEPRINT Translational Research Group, Ottawa Hospital Research Institute.
Data sharing: The Canadian Community Health Survey: Public Use Microdata File, 2017/2018 is available upon request from Statistics Canada.
- Accepted December 14, 2022.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/