Abstract
Background: Hysterectomy, the most common gynecological operation, requires surgeons to counsel women about their operative risks. We aimed to develop and validate multivariable logistic regression models to predict major complications of laparoscopic or abdominal hysterectomy for benign conditions.
Methods: We obtained routinely collected health administrative data from the English National Health Service (NHS) from 2011 to 2018. We defined major complications based on core outcomes for postoperative complications including ureteric, gastrointestinal and vascular injury, and wound complications. We specified 11 predictors a priori. We used internal–external cross-validation to evaluate discrimination and calibration across 7 NHS regions in the development cohort. We validated the final models using data from an additional NHS region.
Results: We found that major complications occurred in 4.4% (3037/68 599) of laparoscopic and 4.9% (6201/125 971) of abdominal hysterectomies. Our models showed consistent discrimination in the development cohort (laparoscopic, C-statistic 0.61, 95% confidence interval [CI] 0.60 to 0.62; abdominal, C-statistic 0.67, 95% CI 0.64 to 0.70) and similar or better discrimination in the validation cohort (laparoscopic, C-statistic 0.67, 95% CI 0.65 to 0.69; abdominal, C-statistic 0.67, 95% CI 0.65 to 0.69). Adhesions were most predictive of complications in both models (laparoscopic, odds ratio [OR] 1.92, 95% CI 1.73 to 2.13; abdominal, OR 2.46, 95% CI 2.27 to 2.66). Other factors predictive of complications included adenomyosis in the laparoscopic model, and Asian ethnicity and diabetes in the abdominal model. Protective factors included age and diagnoses of menstrual disorders or benign adnexal mass in both models and diagnosis of fibroids in the abdominal model.
Interpretation: Personalized risk estimates from these models, which showed moderate discrimination, can inform clinical decision-making for people with benign conditions who may require hysterectomy.
Hysterectomy is one of the most frequently performed surgical procedures. Canada has one of the highest rates of hysterectomy globally, with one-third of women undergoing this procedure before 60 years of age.1 Minimal access approaches are favoured by both clinicians and patients,2 and the proportion of hysterectomies being undertaken by a laparoscopic approach has increased substantially in many countries over the last 10 years.3–7 The evidence-based medicine paradigm for surgical approaches to hysterectomy for benign disease advocates that the chosen surgical approach should be discussed with the patient by their surgeon and decided in light of the relative benefits and risks.2 This advice is echoed by national guidelines.8,9
Most clinicians undertaking hysterectomy will intuitively identify patient characteristics that have the potential to increase the complexity and complications of surgery. A 2016 systematic review of studies that reported significant associations between patient characteristics and surgical outcomes for laparoscopic hysterectomy and a 2020 population-based prospective cohort study using data from the Danish hysterectomy database have suggested that older age, race, raised body mass index (BMI), diabetes mellitus, increased uterine weight, fibroids, endometriosis and adhesions are predictors of complications in patients undergoing hysterectomy for benign indications.10,11 However, assimilating this information to individualize and anticipate the precise risk for each patient if there are multiple factors present can be challenging. A 2020 systematic review reported that surgeons in other specialties were outperformed by risk prediction models in estimating postoperative risk and outcomes; their discriminatory ability showed greater variation (C-statistic 0.51–0.75) than other risk prediction tools.12
Patients should be given information about potential risks before surgery to manage expectations.13 This is especially important when surgery is considered for benign disease because nonsurgical options are often available.
Our aim was to generate prediction models that can be used in conjunction with a surgeon’s intuition to enhance preoperative patient counselling and match the advances made in the technical aspects of surgery. We sought to quantify the proportion of patients who underwent hysterectomy for benign disease and will have a major complication, and to develop and validate prognostic models to individualize this risk, using a national data set.
Methods
Study design, data source and participants
We developed and validated multivariable logistic prediction models using routinely collected data in a retrospective cohort study. Women with benign gynecologic conditions in England are initially assessed by their primary care physician and may be referred to hospital to see a gynecologist if medical treatment is unsuccessful. Hysterectomies for benign disease are performed in most hospitals, and there are regional centres for women with complex benign disease. Hospital Episode Statistics (HES) is an administrative database that holds records of all inpatient admissions in the English National Health System (NHS). Each admission is given a primary diagnosis and up to 20 secondary diagnoses, which are categorized using the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10), and up to 24 procedure fields coded using the Office of Population Censuses and Survey’s Classification of Surgical Operations and Procedures, 4th revision (OPCS-4). For the development and validation of our prognostic models, we identified all patients undergoing a laparoscopic or abdominal hysterectomy for benign reasons between January 2011 and December 2018. Data extraction and analyses were carried out in accordance with relevant guidelines and regulations.
We identified patients undergoing hysterectomy using the OPCS-4 codes Q07 (abdominal excision of uterus) and Q08 (vaginal excision of uterus). We excluded the following: women who had a primary diagnosis of malignant disease; women with a primary diagnosis of female genital prolapse, as a vaginal approach is preferred in these women and those undergoing open or laparoscopic approaches for this diagnosis are likely to have concomitant procedures for prolapse at the time of hysterectomy that would carry additional and specific complication risks; women who underwent a robotic approach, as the uptake of robotic surgery for benign disease in England is limited; and women who underwent vaginal hysterectomy for benign nonprolapse disease (a 2019 restrospective cohort analysis involving women who underwent hysterectomies in England reported that this accounts for less than 2.8% of women who underwent hysterectomy for benign nonprolapse disease).3 We also excluded patients who were younger than 18 years of age or who had missing data for age, duplicated cases and those that had more than 2 major complications on the basis that these were likely to be coding errors.
We identified patients having a laparoscopic hysterectomy when the Q07 or Q08 codes were combined with the Y75 code (minimal access to abdominal cavity), Y50.8 code (approach through abdominal cavity, other specified) and Y71.4 code (failed minimal access approach converted to open). We included failed minimal access procedures in the laparoscopic group because this was the intended route of surgery. This is a prognostic model designed for use preoperatively and, therefore, is an “intention-to-treat” type of analysis. The laparoscopic codes that we used in this study are a broader set of codes than those suggested by the National Institute for Health and Care Excellence (NICE)14 because these codes are more likely to capture laparoscopic procedures and reflect the changes in coding from when the NICE guidance was published.
The codes we chose to identify the route of hysterectomy were published previously from HES data.3 A full list of procedure codes and excluded diagnostic codes can be found in Appendix 1, Supplementary Tables 1 and 2, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.220914/tab-related-content.
Outcome
We used a composite primary outcome of major surgical complications according to the Clavien–Dindo classification, the internationally accepted core outcomes for postoperative complications. We included ureteric, gastrointestinal and vascular injury, and wound complications requiring operative treatment identified either in the index admission or in any hospital admission in the 28 days after surgery. We also included any reoperation after the index procedure on any subsequent admission within 28 days after surgery and other serious complications including shock, renal failure and external resuscitation. These major complications are comparable to the modified Clavien–Dindo classification grades III–IV.15,16 In constructing this composite outcome, we considered the recommendations that these outcomes be of similar importance and occur with a similar frequency with the assumption that the direction of the association of each of these outcomes used to formulate the composite outcome was the same for each predictor;17 these assumptions are supported by the existing literature.2,18–20 The classifications can be found in Appendix 1, Supplementary Tables 3 and 4. A full list of the OPCS-4 and ICD-10 codes and their description are presented in Appendix 1, Supplementary Table 5.
Predictors
We selected 11 predictors for inclusion in the models on the basis that the information would be readily available in the preoperative setting, and based on research describing factors associated with complications.21–28 Age, BMI, diabetes and indication for surgery have all been shown to influence complications.11,25,27,29–31 Uterine weight has also been shown to influence outcomes;11,29,32 however, we did not include it because it would not be available preoperatively. The gynecologic diagnoses were chosen as they represent most benign indications at the time of hysterectomy. Preoperative patient characteristics included the age of the patient, which was the only continuous predictor we included. We categorized ethnicity as follows: white, Black African and Caribbean (including African, Caribbean and any other Black background), Asian (including Indian, Pakistani, Bangladeshi, Chinese and any other Asian background), and other and unknown (including any mixed background). The HES database requires patients to self-identify their ethnicity in 16 categories conforming with the 2001 census classification. We further categorized this in line with the 2021 census. Ethnicity has been shown to be an independent factor influencing the route and complications of hysterectomy.3,25,27,30,33–37 Our categorization of ethnicity is more detailed than the previous model available.29
We identified clinical predictors for conversion from ICD-10 codes for obesity and diabetes.31 Common gynecologic diagnoses recorded at the time of hysterectomy were identified from ICD-10 codes and include fibroids, menstrual disorders, endometriosis and pain, adenomyosis and benign adnexal mass. Women may have more than 1 gynecologic diagnosis at the time of hysterectomy.38 We used adhesions as a proxy of a previous history of abdominal surgery, since 90% of adhesions occur because of previous open abdominal surgery.39 The presence of intra-abdominal adhesions or concomitant adhesiolysis were identified from OPCS-4 or ICD-10 codes. These specific codes have not been validated, although they have been used in several previous analyses.3,19,31,33,40,41 Previous validation studies of HES coding have shown acceptable reliability,42–44 and a 2012 systematic review reported that the the accuracy of diagnostic coding using HES data was 83%–96%.45 A full list of codes and their description that we used to formulate these predictors can be found in Appendix 1, Supplementary Table 6.
Model development
Our model development and validation process followed current recommendations with regard to the selection and coding of predictors, the specification and estimation of the model, as well as the predictive performance assessment measures and model presentation.46 We hypothesized that heterogeneity among populations and uptake of laparoscopic approaches between geographic regions may contribute to differences in model performance.3,47 Therefore, we divided the data into 8 different NHS regions. Seven regions were used in model development and internal–external cross-validation (Northern and Yorkshire, Trent, West Midlands, North West, Eastern, South East and South West). The eighth region (London) was not used in model development but was used for further validation, independent of the model development cohort. We chose London for validation as we hypothesized it was the most diverse region when all factors were considered, and would allow a robust test for generalizability.48 Hospital Episode Statistics data have been used previously to develop prediction models.33,47 We used a multivariable logistic regression modelling approach with prespecified predictors and did not use a selection strategy. We assessed nonlinear association between age and major complications using fractional polynomials, quadratic terms and a step function, and we decided to model age as a quadratic term (Appendix 2, Supplementary Figure 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.220914/tab-related-content). Imputation of missing values was not necessary because more than 99.9% of the patients in the data set had complete data.
Model validation
During validation, we assessed model discrimination (how well predictions differentiated those who had a major complication from those who did not, quantified as the C-statistic), calibration (agreement between predicted and observed risk, assessed using calibration slopes), calibration-in-the-large (average predicted number of outcome events compared with number of observed outcome events) and calibration plots. An ideal calibration slope is 1, while calibration-in-the-large should ideally be 0.
We validated the model in the development cohort first using an internal–external cross-validation framework to concurrently evaluate between-region heterogeneity and assess generalizability.46,49 In this process, each of the 7 contributing NHS regions were iteratively excluded from the development set; the model was then trained using the prespecified predictors in the remaining regions and validated in the omitted region by quantifying the C-statistic, calibration slope and calibration-in-the-large statistics across development regions. We used random-effects meta-analysis to calculate pooled C-statistics, calibration slopes and calibration-in-the-large statistics across development NHS regions. We evaluated forest plots to assess between-region heterogeneity. The final model was validated further in using data from the London NHS region, which had been held out from model development.
Model presentation
We developed user-friendly online calculators for both models and, in addition, we developed graphical calculators (nomograms) for both models.
We considered aspects of Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD) in the reporting of our findings.50 We used Stata software version 16.1 to perform the analyses.
Ethics approval
This research involved only previously collected nonidentifiable data and, therefore, did not require review by a United Kingdom research ethics committee. There was no patient or public involvement in this research. All data extraction and analyses were carried out in accordance with relevant national guidelines and regulations. Only aggregated totals of patients and procedures are reported, and no identifiable information was available for analysis.
Results
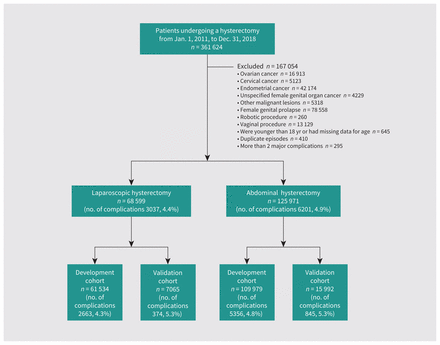
Between Jan. 1, 2011, and Dec. 31, 2018, 361 624 patients underwent hysterectomy in English NHS hospitals. We excluded 69 528 patients with a primary diagnosis of malignant disease and 78 558 with a primary diagnosis of female genital prolapse, 260 who had a robotic hysterectomy and 13 129 who underwent a vaginal hysterectomy for benign (nonprolapse) disease. We also excluded 645 people who were younger than 18 years of age or had missing data for age, 295 with more than 2 major complications and 410 duplicate episodes. This left 68 599 patients who had laparoscopic hysterectomies, 3307 (4.4%) of whom had a major complication, and 125 971 patients who had an abdominal hysterectomy, 6201 (4.9%) of whom had a major complication (Figure 1).
Flow chart of the study population. We identified patients from the Hospital Episode Statistics database to develop and validate prediction models for major complications in patients undergoing laparoscopic or abdominal hysterectomy for benign disease.
We found that the number of laparoscopic hysterectomies increased, and the number of abdominal hysterectomies reduced over time. The number of major complications stratified by region and time are shown in Table 1. We included 61 534 patients who underwent laparoscopic and 109 979 who underwent abdominal hysterectomies in the 7 regions used in our model development. We held out a further 7065 patients who underwent laparoscopic and 5356 who underwent abdominal hysterectomies from 1 region (London) for additional validation (Table 1). A detailed breakdown of the specific type of complications stratified by year and region are shown in Appendix 1, Supplementary Tables 7 and 8.
Number of patients undergoing hysterectomy, by surgical route, year and region of England, and number and percentage of major complications within 28 days, by surgical route and year
Candidate predictors stratified by development and validation cohorts and univariate analysis of the association of prognostic factors from the development cohort associated with major complications are shown in Table 2. In univariate analysis of both routes of hysterectomy, we found that adhesions had the strongest association with major complications, with more than double the odds (laparoscopic odds ratio [OR] 2.03, 95% confidence interval [CI] 1.87 to 2.20; abdominal OR 2.50, 95% CI 2.35 to 2.65).
Candidate predictors stratified by route of hysterectomy and development and validation data sets with crude odds ratios of potential prognostic determinants of a major complication in the development data set
In the model for a laparoscopic approach, we found that menstrual disorders (adjusted OR 0.75, 95% CI 0.69 to 0.82), benign adnexal masses (adjusted OR 0.85, 95% CI 0.77 to 0.94) and other gynecologic diagnoses at the time of hysterectomy (adjusted OR 0.87, 95% CI 0.79 to 0.96) were protective against major complications (Table 3). Adenomyosis (adjusted OR 1.46, 95% CI 1.36 to 1.60) and adhesions (adjusted OR 1.92, 95% CI 1.73 to 2.13) were associated with increased risk of major complications. In the model for an open abdominal approach, we found that fibroids (adjusted OR 0.75, 95% CI 0.71 to 0.80), menstrual disorders (adjusted OR 0.52, 95% CI 0.48 to 0.55), benign adnexal masses (adjusted OR 0.79, 95% CI 0.74 to 0.84) and other gynecological diagnoses (adjusted OR 0.78, 95% CI 0.73 to 0.85) were protective against major complications. We also found that Asian ethnicity (adjusted OR 1.40, 95% CI 1.24 to 1.58), diabetes (adjusted OR 1.16, 95% CI 1.03 to 1.30) and adhesions (adjusted OR 2.46, 95% CI 2.27 to 2.66) were associated with increased risk of major complications. In both models, adhesions was the strongest predictor of complications. The apparent C-statistic of the laparoscopic model (discriminatory ability) was 0.60 (95% CI 0.60 to 0.62), and the abdominal model 0.67 (95% CI 0.65 to 0.69). The full model coefficients are shown in Appendix 1, Supplementary Table 9, to enable independent model reconstruction.
Multivariable logistic regression models for prediction of major complications for each of the routes of hysterectomy*
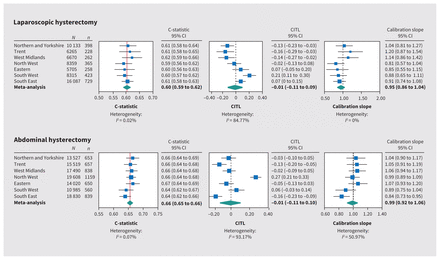
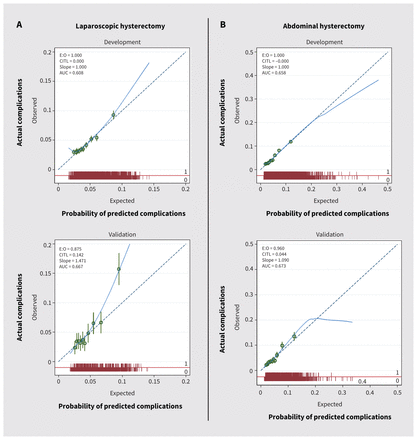
Forest plots showing model discrimination (C-statistic) and calibration metrics (slope and calibration-in-the-large) from internal–external cross validation49 of both prognostic models in the development cohort are shown in Figure 2. Visual calibration plots for development and validation cohorts for both models are shown in Figure 3, and calibration plots by development NHS region are shown in Appendix 3, Supplementary Figure 3A and 3B, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.220914/tab-related-content.
Internal–external cross validation of laparoscopic and abdominal models, by National Health Service region. Broken lines indicate lines of perfect calibration-in-the-large (0) and calibration slope (1); blue squares indicate point estimates; bars indicate 95% CIs; and diamonds indicate estimates from random-effects meta-analysis. Note: CI = confidence interval, CITL = calibration-in-the-large.
Calibration plots for prediction of major complications in patients undergoing (A) laparoscopic or (B) abdominal hysterectomy for benign indications. Note: AUC = area under the curve, CITL = calibration-in-the-large, E = expected, O = observed.
In the laparoscopic model, we found that C-statistics were consistent across development regions (point estimates 0.59 to 0.62; pooled random-effects meta-analysis estimate 0.60, 95% CI 0.59 to 0.62). Calibration slopes showed minor heterogeneity across regions (point estimates 0.81 to 1.20; pooled estimate 0.95, 95% CI 0.86 to 1.04). There was some heterogeneity across regions in calibration-in-the-large (point estimates −0.16 to 0.21; pooled estimate −0.01, 95% CI −0.11 to 0.09). Overall risk was overestimated in Northern and Yorkshire, Trent and West Midlands, and underestimated (the actual risk is higher than the predicted risk) in the South West. We validated the final laparoscopic prognostic model, trained in the development cohort, in the held-out NHS region. The C-statistic was 0.67 (95% CI 0.64 to 0.70), calibration-in-the-large 0.14 (95% CI 0.04 to 0.25) and calibration slope 1.47 (95% CI 1.24 to 1.70).
In the abdominal model, we found that C-statistics were also consistent across development regions (point estimates 0.64 to 0.67; pooled random-effects meta-analysis estimate 0.66, 95% CI 0.65 to 0.66). Calibration slopes showed minor heterogeneity (point estimates 0.84 to 1.07; pooled estimate 0.99, 95% CI 0.92 to 1.06). There was some heterogeneity across regions in calibration-in-the-large (point estimates −0.16 to 0.27; pooled estimate −0.01, 95% CI −0.11 to 0.10). Overall risk was overestimated (predicted risk is higher than the actual risk) in Trent and the South East and underestimated in the North West. We validated the final abdominal prognostic model, trained in the development cohort, in the held-out NHS region. The C-statistic remained stable at 0.67 (95% CI 0.65 to 0.69); calibration-in-the-large was 0.04 (95% CI −0.03 to 0.11) and calibration slope was 1.09 (95% CI 0.97 to 1.21).
The online calculator can be found at www.evidencio.com (laparoscopic, https://www.evidencio.com/models/show/2551; abdominal, https://www.evidencio.com/models/show/2552). The models are also presented as nomograms in Appendix 4, Supplementary Figure 4, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.220914/tab-related-content. Examples of how to use the nomograms are also shown in Appendix 5, Supplementary Figure 5, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.220914/tab-related-content.
Interpretation
Our prognostic models for major complications in laparoscopic and abdominal hysterectomy had acceptable predictive ability.51 Our internal–external cross-validation and external validation showed moderate discrimination. The final models integrate 11 routinely available predictors and are intended for use when counselling patients preoperatively. The models are relevant for gynecologists to aid preoperative counselling and to individualize risk using the calculator. This tool is not applicable to patients undergoing hysterectomy for malignant disease.
We have used a large national multiinstitutional database with full coverage of English NHS hospitals, which enhances generalizability. Therefore, the estimation of the rate of major complications is precise and representative of national practice. We are unaware of any other prediction models for major complications in patients undergoing abdominal hysterectomy for benign disease. Our prediction model for laparoscopic hysterectomy was developed in twice the number of patients of an existing model.29 This robust sample links patients by a unique identification number that allowed patients who were admitted to a different hospital in the postoperative period to be identified and linked to the index episode, therefore minimizing loss to follow-up. The coding for age and primary diagnosis in this database has been shown to be accurate, and the accuracy of reporting for comorbidities such as obesity and diabetes have improved.45,52 The HES data have been used previously to produce prediction models.33,47
The most significant risk factor for major complications in both models was the presence of adhesions, which is consistent with existing literature.11,20,24,26 Adhesions should be suspected if there is a previous history of laparotomy,39 cesarean section,53 pelvic infection or endometriosis,2,39 and can be reliably diagnosed preoperatively using ultrasonography.54,55 As the global rate of cesarean sections continues to rise, this will undoubtedly remain a key determinant of major complications.
We found that patients of Asian descent were at higher risk of major complications after abdominal hysterectomy than patients who were white; however, we did not find that race was a predictor for complications in laparoscopic hysterectomy as reported in previous studies.25,27,28 Previous studies have shown that patients who were not white, in particular patients of Asian descent,37 are less likely to undergo a minimal access approach,27,34,36 and this disparity in care merits further investigation.
In our model, obesity was not a significant predictor of major complications for either route of hysterectomy. We may be criticized for using a binary variable rather than exact BMI; however, the existing model for prediction of complications in laparoscopic hysterectomy used exact BMI as a predictor but did not find BMI to be a statistically significant predictor.29 Comparison of this finding with existing literature is challenging in view of the heterogeneity between studies in the definition of complications. A 2020 population-based prospective cohort study in Denmark using multi-institutional data found that obesity was a significant predictor of complications; however, the study included deep vein thrombosis as a major complication.11 A 2013 longitudinal observation study in Finland did not find obesity to be a risk factor for major complications; however, it did report that obesity increased the risk of post-operative infections, which would not be included in our composite outcome if managed by nonsurgical measures.4 A 2021 study of the effects of obesity on peri- and postoperative outcomes in patients undergoing robotic versus conventional hysterectomy that involved women undergoing a total hysterectomy for benign indications in Sweden reported longer operating times and longer hospital stays but no significant difference in reoperation or readmission; however, robotic surgery was included.56 A 2016 systematic review of factors associated with outcomes in laparoscopic hysterectomy concluded that a BMI of more than 30 influenced operating times and blood loss (greater),10 which were not included in our composite outcome. A prospective, multi-institutional, risk-adjusted cohort study involving 118 707 patients undergoing nonbariatric general surgery found overall morbidity to be lower in patients with a normal weight: a phenomenon known as the obesity paradox.57 This is thought to be because there are subsets of patients who are obese: those who have metabolic disturbances and those who do not. There is evidence that obese patients with metabolic syndrome (specifically diabetes and hypertension) who undergo general, vascular and orthopedic surgery are at increased risk of morbidity and mortality than those in the normal weight range.58 We included diabetes as a separate variable, and obese patients with metabolic disturbance may have been captured in this group. Although we found no evidence that obesity is a factor influencing the incidence of major complications, surgeons who use this tool must be aware that the outcome does not include venous thromboembolism or wound complications not requiring surgical intervention for which there is existing evidence.59 We found that diabetes did not have an impact on major complications in laparoscopic surgery as found in a previous study31 but did have an impact on outcomes for abdominal hysterectomy, in keeping with previous reports of increased adverse outcomes after orthopedic and ear, nose and throat surgery, including cardiac complications and intensive care admissions.58,60–64 With the prevalence of diabetes rising, it is an important factor to consider.65
Previous studies have shown that higher uterine weight is a predictor of major complications,11,29 and 1 study reported that, although uterine weight was a risk factor for complications, abdominal hysterectomy had higher odds of complication than laparoscopic hysterectomy for all strata of weight.32 We used a diagnosis of fibroids as a proxy of uterine weight and found that this was not a significant predictor of complications in the laparoscopic model and was a protective factor in the abdominal model, which was an unexpected finding. This may be because fibroids are coded as a diagnosis even when they do not significantly increase uterine weight or are coded to justify an open approach. However, there may be reasons beyond these that must be considered. In 2014, the U.S. Food and Drug Administration issued a statement discouraging the use of power morcellation in patients undergoing hysterectomy for fibroids because these patients may have occult leiomyosarcoma and morcellation would spread the tissue and worsen the prognosis.66 In the United States, the proportion of hysterectomies performed abdominally for patients with fibroids increased at the expense of laparoscopic procedures.67 It is plausible that owing to these controversies, surgeons in the NHS may have opted for an open approach in those with fibroids that were not substantial in size and who had few other risk factors who may have been otherwise suitable for a laparoscopic approach. The UK has also been criticized for being slow to adopt laparoscopic approaches to hysterectomy because of restrictive national guidance,68 and perhaps this finding highlights that patient selection for minimal access approaches for patients who have fibroids is more timid than in other countries.
Limitations
Despite the large cohort, our study has limitations, including the lack of detailed clinical information on exact BMI, location, type and size of leiomyoma, or severity of adhesions and endometriosis, and the long-term outcome of complications. Large databases may also have coding errors. The discriminatory ability of our tools falls a little below what would usually be considered “good” (0.7), and this may be due to the age and the granularity of the data. The database does not include information on the experience or training of surgeons, which has been shown to influence complications rates.69,70 Our calibration of the model was optimal, with CIs overlapping the reference ideal line; however, in the highest risk decline group undergoing laparoscopic hysterectomy, the probability predicted by the model underestimated the actual risk and, in the highest risk decline group undergoing abdominal hysterectomy, the model overestimated the actual risk.
Conclusion
We have developed simple online prediction tools using routinely collected data that provide personalized risk estimates for patients undergoing hysterectomy for benign disease and can be used by surgeons to aid preoperative counselling. These tools will guide shared decision-making and may lead to referral to centres with greater surgical expertise or to exploration of nonsurgical treatment options. Although a surgeon’s experience and expert opinion carries utility, it cannot be used solely to guide risk management. In Canada and globally, the overall rate of hysterectomy for benign disease is declining, and more patients are undergoing surgery by lower-volume surgeons, who may not have expertise in every procedure. Most hysterectomies in Canada are for benign indications and, with calls for ongoing investment into gynecologic surgery, our models could be useful tools to stratify risk. Further research should focus on improving the discriminatory ability of these tools by including factors other than patient characteristics, including surgeon volume, as this has been shown to reduce complications.
Footnotes
Competing interests: Krupa Madhvani has received article processing fees from Elly Charity (East London International Women’s Health Charity). No other competing interests were declared.
This article has been peer reviewed.
Contributors: Krupa Madhvani conceived the study. Krupa Madhvani and Tyrone Carpenter acquired the data. Krupa Madhvani, Tyrone Carpenter and Khalid Khan interpreted the data. Silvia Fernandez Garcia, Borja Fernandez-Felix and Javier Zamora analyzed the data. Krupa Madhvani and Khalid Khan drafted the manuscript. All of the authors designed the study, revised the manuscript for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: The acquisition of the data was funded by the British Society for Gynaecological Endoscopy. They were not involved in the study design, analysis, interpretation of data, the writing of the report or the decision to submit the article for publication. Khalid Khan is a Distinguished Investigator funded by the Beatriz Galindo (senor modality) Program grant given to the University of Granada by the Ministry of Science, Innovation, and Universities of the Government of Spain.
Data sharing: National Health Service Digital provided pseudo-anonymized data under Data Sharing Framework Contract CON-86057-F2D7Q and Data Sharing Agreement NIC-82980-V6D4Q. The terms of the licence do not permit the authors to make the data publicly available, but the data can be requested via formal application to NHS Digital using its online Data Access Request Service (http://content.digital.nhs.uk/dars).
- Accepted September 4, 2022.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/