Abstract
Background: Accidental acetaminophen overdoses are associated with substantial morbidity and health care costs. In Canada, updated labelling standards were implemented in October 2009 and September 2016, with the intent of communicating risks of overdose and facilitating product identification and safe use, respectively. Full compliance with the 2016 standards was expected by March 2018. We sought to explore whether these changes affected rates of hospital admission for accidental acetaminophen overdose.
Methods: We conducted a population-based study of hospital admissions for accidental acetaminophen overdose in 9 Canadian provinces and 3 Canadian territories between Apr. 1, 2004, and Mar. 31, 2020. We used interventional autoregressive integrated moving average (ARIMA) models to evaluate the impact of the updated labelling standards on rates of hospital admission for accidental acetaminophen overdose. In secondary analyses, we studied intensive care unit (ICU) admissions and hospital admissions for accidental acetaminophen overdose involving opioids.
Results: Monthly rates of hospital admission for accidental acetaminophen overdose were essentially unchanged over the study period (0.21 and 0.22 cases per 100 000 population in April 2004 and March 2020, respectively). We found no association between changing labelling standards and trends in rates of hospital admission for accidental acetaminophen overdose (October 2009 p = 0.2, September 2016 p = 0.7 and March 2018 p = 0.2). Similarly, labelling changes did not have an impact on admissions involving ICU admission and concomitant opioid poisoning.
Interpretation: Modifications to product labels did not reduce the rate of acetaminophen-related harm. Additional measures to reduce the burden of accidental acetaminophen overdose are required.
A cetaminophen is used by millions of people worldwide.1–4 Although generally safe at therapeutic doses, acetaminophen overdose is the leading cause of acute liver failure in several countries, including Australia, the United Kingdom and the United States, where it represents 50% of all reported cases.5–8 Although most acetaminophen overdoses are a result of deliberate self-harm, accidental overdoses represent a substantial and increasing proportion of these events.8–10 In Canada, the proportion of acetaminophen-related injuries from accidental overdose increased from 27% in 2006 to 45% in 2011.11 Factors contributing to unintentional overdose include a lack of consumer awareness regarding the presence of acetaminophen in nonanalgesic over-the-counter products, unclear dosing instructions on product labels and the simultaneous ingestion of prescription and nonprescription medications containing acetaminophen.8,12
Because accidental acetaminophen overdose is a potentially fatal yet avoidable form of drug-related harm, initiatives for risk minimization have been implemented in various jurisdictions such as restricting package size and changing package configurations to blister packs.10 In Canada, measures to mitigate the risk of inadvertent overdose have involved changes to product labels on 2 separate occasions (Appendix 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.210842/tab-related-content). Specifically, in late October 2009, labelling standards were modified to require increased warnings about the potentially fatal risk of liver injury in the event of acetaminophen overdose.13 An updated labelling standard, intended to facilitate product identification and communicate safe dosing, was introduced in September 2016.14 These standards were required immediately for new products, with full compliance for all products expected by March 2018.
At their time of implementation, these labelling changes attracted substantial media interest and generated debate regarding their potential effectiveness.15–18 One study showed that the changes to acetaminophen labels mandated by the US Food and Drug Administration could improve consumer perception of the risk of hepatotoxicity, but the effectiveness of label changes in reducing accidental acetaminophen overdoses is unknown.19 Moreover, although 1 Canadian study found that emergency department visits for acetaminophen toxicity declined between 2011 and 2018, findings from that study were generated from a nonrepresentative sample comprising mostly pediatric hospitals in large urban centres.20 In addition, the study did not explore the impact of labelling changes on accidental acetaminophen overdose. Therefore, we sought to evaluate whether the implemented changes in product labelling were associated with reduced rates of hospital admission for accidental acetaminophen overdose in Canada.
Methods
Setting
We conducted a population-based study of hospital admissions for accidental acetaminophen overdose in 9 Canadian provinces and 3 Canadian territories between Apr. 1, 2004, and Mar. 31, 2020.
Data sources
We identified hospital admissions for accidental acetaminophen overdose using the Canadian Institute for Health Information’s Discharge Abstract Database, which includes clinical and demographic information on inpatient admissions for all Canadian provinces and territories, excluding Quebec. Emergency department visits that do not culminate in hospital admission are not included in the database.
Study population and outcomes
For each month of the study period, we defined our study population of people at risk for accidental acetaminophen overdose as all residents of any age residing within the 12 Canadian jurisdictions included in the study. Our primary outcome was the monthly rate of hospital admissions for accidental acetaminophen overdose. Specifically, we first defined all admissions for acetaminophen overdose as inpatient stays with diagnosis code T39.1, from the International Classification of Diseases, 10th revision (ICD-10), at the time of admission. The positive predictive value of this code for acetaminophen overdose was 95% in an earlier study.21 Next, we designated acetaminophen overdoses as accidental using codes for “external causes of injury” (i.e., ICD-10 codes X40 and Y45.5). In secondary analyses, we evaluated quarterly rates of admission to the intensive care unit (ICU) for accidental acetaminophen overdose and quarterly rates of accidental acetaminophen overdose involving concomitant opioid poisoning (ICD-10 codes T40.2, T40.4, X42).
Statistical analysis
We used interventional autoregressive integrated moving average (ARIMA) models to evaluate the impact of labelling changes on rates of hospital admission for accidental acetaminophen overdose.22,23 We used the Dickey–Fuller test to determine the stationarity of the time series, and applied first-order differencing to arrive at a stationary series, if needed.23,24 We used the autocorrelation function and partial autocorrelation function to identify autoregressive or moving average components in each time series and correct for autocorrelation remaining after differencing, and selected the best models using goodness-of-fit tests.22,23 We used residual plots and the Portmanteau statistic to confirm that residuals from specified ARIMA models were a white-noise process.22,23,25 Finally, once the ARIMA models were specified, we used ramp intervention functions to test for gradual changes in trends of each outcome after the October 2009 labelling changes (modelled as a November 2009 intervention point), the September 2016 updated labelling standard and the March 2018 deadline for compliance with the September 2016 standards for all acetaminophen products (see Appendix 2 for final models, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.210842/tab-related-content).23 Ramp transfer functions take the value of 0 before the intervention and increase in integer increments for each period after the intervention. We selected ramp transfer functions rather than step functions to model our intervention because we hypothesized that any impacts of labelling changes on admissions for accidental acetaminophen overdose would manifest gradually rather than suddenly, given the nature of the interventions.
We conducted several analyses to test the robustness of our findings. We tested for stability in each time series by plotting the cumulative sums of ordinary least squares residuals (OLS–CUSUM) and corresponding confidence bands over the study period.26,27 A structural break is identified by movement of the plotted CUSUM beyond the confidence bands and the associated test statistic. For outcomes where the OLS–CUSUM test suggested the presence of structural changes, we used the Bai–Perron test to identify the dates of endogenous structural breaks.28 We also used Chow tests to evaluate whether each labelling intervention point was associated with shifts in the ensuing level and slope of the time series.29 We completed all analyses using SAS Enterprise Guide, version 6.1 (SAS Institute Inc.), R Studio and Stata version 17.0 (Stata Corp LP).
Ethics approval
The study was approved by the Research Ethics Board of Unity Health Toronto (no. 19-208).
Results
During the 16-year study period, we identified 12 212 hospital admissions for accidental acetaminophen overdose, of which 2267 (18.6%) required ICU admission and 2245 (18.4%) were complicated by concomitant opioid overdose (Table 1). The median age of the patients was 39 (interquartile range 22–56) years and most patients were female (n = 7606, 62.3%) (Table 1). Overall, 2092 (17.1%) patients had comorbid alcohol use disorder and 1333 (10.9%) had chronic liver disease (Table 1).
Demographic and clinical characteristics of patients admitted to hospital with accidental acetaminophen overdose, 2004–2005 to 2019–2020
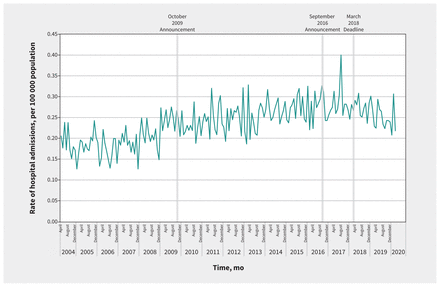
In our primary analysis, the monthly rate of hospital admission for accidental acetaminophen overdose was 0.21 per 100 000 population (n = 50 patients) in April 2004 and 0.22 per 100 000 population (n = 64 patients) in March 2020 (Figure 1). The average monthly percent change in admissions for accidental acetaminophen overdose for the entire study period was 0.2% (95% confidence interval [CI] 0.1% to 0.2%). The average monthly percent change for each segment of the time series (i.e., baseline to October 2009, November 2009 to August 2016, September 2016 to February 2018 and March 2018 to March 2020) was 0.3% (95% CI 0.3% to 0.4%), 0.3% (0.3% to 0.4%), −0.4% (−0.5% to 0.1%) and −0.4% (−0.9% to −0.1%), respectively. Using interventional ARIMA modelling, we found no significant change in the trend of rates of hospital admission for accidental acetaminophen overdose after the October 2009 labelling standard (p = 0.2) (Figure 1). Similarly, we did not observe a change after the updated labelling standard in September 2016 (p = 0.7) and the March 2018 deadline for full compliance with this standard (p = 0.2) (Figure 1). In sensitivity analyses, we identified a structural break before the implementation of labelling changes (December 2008, p < 0.001), with no labelling intervention dates identified as structural breaks (Appendix 4 and Appendix 5, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.210842/tab-related-content).
Monthly rates of hospital admissions for accidental acetaminophen overdose in Canada (excluding Quebec), April 2004 to March 2020.
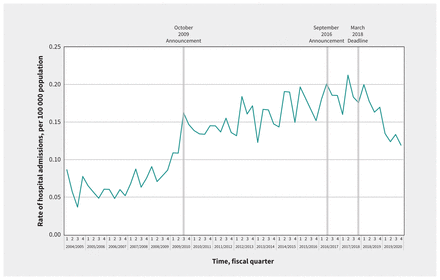
In our secondary analysis, quarterly rates of ICU admissions for accidental acetaminophen overdose were 0.12 per 100000 population (n = 29 patients) in the first quarter of 2004 and 0.14 per 100 000 population (n = 41 patients) in the final quarter of 2019 (Figure 2). The average quarterly percent change in this outcome was 0.7% (95% CI 0.2% to 1.1%). The average quarterly percent change for each segment of the time series was 1.4% (95% CI 1.0% to 2.2%), 0.8% (0.3% to 1.4%), −1.1% (−3.3% to 1.0%) and −1.1% (−6.3% to 0.4%), respectively. We did not observe a significant change in the trend of ICU admissions for accidental acetaminophen overdose after the October 2009 (p = 0.2), September 2016 (p = 0.2) or March 2018 (p = 0.8) interventions (Figure 2). We observed similar findings in sensitivity analyses, with no structural breaks identified using the OLS-CUSUM (Appendix 4) and Chow tests (Appendix 5).
Quarterly rates of admission to the intensive care unit (ICU) for accidental acetaminophen overdose in Canada (excluding Quebec), April 2004 to March 2020.
Rates of hospital admissions for accidental acetaminophen overdose involving opioids doubled between the first quarter of 2004 (0.09 per 100 000 population; n = 21 patients) and the quarter immediately preceding the March 2018 deadline for compliance with labelling changes (0.18 per 100 000; n = 52 patients). The average quarterly percent change over the entire study period was 0.8% (95% CI 0.4% to 1.2%). The average quarterly percent change for each segment of the time series was 3.1% (95% CI 2.1% to 3.8%), 1.1% (0.7% to 1.6%), 1.1% (−3.5% to 1.5%) and −7.3 (−11.9% to −3.5%), respectively. We did not observe a signficant change in trends of hospital admissions for accidental acetaminophen overdose involving opioids after the October 2009 (p = 0.3) or September 2016 (p = 0.9) labelling updates. Although rates of hospital admissions for accidental acetaminophen overdose involving opioids subsequently declined 33.3% by the end of the study period (0.12 per 100 000 population; n = 35 patients), the March 2018 labelling deadline was not associated with this trend (p = 0.07) (Figure 3). However, in sensitivity analyses, we observed a statistically significant step increase in admissions involving opioids after the March 2018 intervention date, followed by a gradual decline in the slope of the time series (Appendix 5). In addition, we identified a structural break in the OLS-CUSUM (Appendix 4) and first quarter of 2016 using the Bai–Perron test.
Quarterly rates of hospital admission for accidental acetaminophen overdose involving opioids in Canada (excluding Quebec), April 2004 to March 2020.
Interpretation
We found that changes to acetaminophen product labels that were intended to optimize the safe use of this drug had no impact on hospital admissions for accidental acetaminophen overdose. Although rates of overdoses involving opioid toxicity declined after the September 2016 labelling update, this finding may reflect a general decline in opioid overdose–related hospital admissions during this period in Canada.30
Our study has important implications for public health and policy. Acetaminophen overdose is the leading cause of acute liver failure in several countries, with accidental overdoses representing a substantial portion of these events.5–9 Moreover, acetaminophen overdoses are associated with considerable cost to the health care system, estimated at more than $87 million annually for deliberate overdoses in the US in 1995.31 Findings from 1 study suggested similar costs in cases of accidental overdose.10 Because of the human and economic burden imparted by accidental acetaminophen overdoses, additional measures for preventing these episodes are required, beyond those that attempt to inform consumers about the potential risks of acetaminophen through product labels and package inserts. This is especially important when considered in light of previous research that showed that fewer than 50% of patients regularly read labelled instructions for use of over-the-counter analgesics,32,33 and only 26% read the active ingredients before first use.33 Further, studies from the US and UK have found that 4.5% to 6% of patients exceed the maximum recommended acetaminophen dose of 4 g/d,34,35 a finding that may be associated with the use of over-the-counter cough and cold preparations that contain acetaminophen, health literacy and high-strength formulations of acetaminophen.36–39 In addition, enhancing the size and visibility of warnings on acetaminophen product labels did not mitigate the risk of supratherapeutic acetaminophen dosing in 1 small study.40
Our findings build on those of earlier studies evaluating interventions intended to mitigate the risk of acetaminophen overdose. Previous research from England and Wales found that acetaminophen poisoning–related mortality declined 43% and liver transplantation registrations owing to acetaminophen-induced hepatotoxicity declined 61% in the 11 years after legislation that restricted acetaminophen package sizes to a maximum of 32 tablets and the amount of acetaminophen that can be sold without a prescription at 1 time to 100 tablets.41 However, the impact of package size restrictions was not uniform throughout the UK, with no impact on fatal poisonings observed in Scotland.42 In Denmark, an 18-year age restriction on over-the-counter analgesic sales was associated with a 17% reduction in admissions for nonopioid analgesic poisoning among people aged 10–17 years, and pack size restriction of these drugs was associated with an 18% reduction in admissions for the entire population.43 However, the age restriction was also associated with a decrease in poisoning associated with other prescription medications, obfuscating a causal association between this policy change and nonopioid analgesic poisonings.43 In contrast to these studies, we explored whether labelling changes alone are sufficient for curbing acetaminophen overdoses. Moreover, previously published studies did not distinguish between accidental overdoses and deliberate self-harm, the latter being the impetus for the legislated restrictions on package sizes, given the often impulsive nature of deliberate overdoses.44 We focused specifically on accidental overdoses, the prevention of which would be the likely goal of labelling changes intended to promote safe use of acetaminophen.
In light of our findings and of the limitations of product labels, implementation and evaluation of measures addressing conditions that predispose people to unintentionally ingest more than the recommended amount of acetaminophen should be considered. Examples of such measures include removing acetaminophen from nonanalgesic over-the-counter products, discontinuing opioid–acetaminophen combination products and restricting products to a maximum of 325 mg of acetaminophen per unit dose.
Limitations
We did not include emergency department visits for accidental acetaminophen overdose that did not result in hospital admission or events resulting in prehospital deaths. Our study may have been underpowered for detecting significant changes, particularly for secondary outcomes with fewer available data points in the later portions of the time series. However, the results of sensitivity analyses were largely aligned with those of ARIMA models, providing support for our main inferences. As with all interrupted time series studies, temporal confounding related to discrete events occurring in close proximity to the interventions of interest or changes in the source population is a potential source of bias. However, we are unaware of any such confounders, and the median age and sex distribution of the national population did not change appreciably between 2004 and 2020.45 Finally, our approach of distinguishing accidental from deliberate acetaminophen overdose has not been validated. However, the age and sex distribution of our patients was similar to those of studies using hospital medical records for identifying cases of accidental acetaminophen overdose.8,10
Conclusion
We found that changes to acetaminophen labels that communicated the risks of overdose and the presence of acetaminophen in over-the-counter products did not affect rates of hospital admission for accidental acetaminophen overdose, ICU admission for accidental acetaminophen overdose and admission for acetaminophen overdoses involving opioids. Given the impacts to the public health and health systems of accidental acetaminophen overdose and the interest of policymakers worldwide in promoting the safe use of acetaminophen, our findings suggest that additional measures are needed for preventing these events.
Acknowledgments
The authors thank Jordan Hunt, Roger Cheng, Trupti Jani and Kathy Lee of the Canadian Institute for Health Information for their assistance in accessing data from the Discharge Abstract Database.
Footnotes
Competing interests: Tara Gomes reports funding from the Canadian Institutes of Health Research and support for committee attendance from Indigenous Services Canada, outside the submitted work. No other competing interests were declared.
This article has been peer reviewed.
Contributors: All authors contributed to the conception of the study and interpretation of findings. Tony Antoniou and Qi Guan conducted analyses. Tony Antoniou drafted the manuscript. All authors revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: Tara Gomes received research support from the Ontario Ministry of Health during the conduct of the study and is supported by a Canada Research Chair in Drug Policy Research and Evaluation.
Data sharing: The data for this study cannot be shared.
Disclaimer: All data were provided by the Canadian Institute for Health Information. However, the analyses, conclusions, opinions and statements expressed herein are those of the authors and not necessarily those of the Canadian Institute for Health Information.
- Accepted February 18, 2022.
This is an Open Access article distributed in accordance with the terms of the Creative Commons Attribution (CC BY-NC-ND 4.0) licence, which permits use, distribution and reproduction in any medium, provided that the original publication is properly cited, the use is noncommercial (i.e., research or educational use), and no modifications or adaptations are made. See: https://creativecommons.org/licenses/by-nc-nd/4.0/