Abstract
BACKGROUND: After diagnosis of a health condition, information about survival and potential transition from community into institutional care can be helpful for patients and care providers. We sought to describe the association between a new diagnosis of dementia and risk of admission to a long-term care home and death at 5 years.
METHODS: We conducted a population-based retrospective cohort study using linked health administrative databases. We identified individuals aged 65 years or older, living in the community, with a first documented diagnosis of dementia between Jan. 1, 2010, and Dec. 31, 2012, in Ontario, Canada. Dementia diagnosis was captured using diagnostic codes from hospital discharges, physician billings, assessments conducted for home care and long-term care, and dispensed prescriptions for cholinesterase inhibitors. Our primary outcome measures were 5-year risk of death and placement in a long-term care home, adjusted for sociodemographic and clinical factors.
RESULTS: We identified 108 757 individuals in our study cohort. By the end of 5 years, 24.4% remained alive in the community and 20.5% were living in a long-term care home. Of the 55.1% who died, about half (27.9%) were admitted to a long-term care home before death. Three risk factors were associated with increased odds of death: older age (age ≥ 90 yr; odds ratio [OR] 9.5, 95% confidence interval [CI] 8.8–10.2 [reference: age 65–69 yr]), male sex (OR 1.7, 95% CI 1.6–1.7), and the presence of organ failure, including chronic obstructive pulmonary disease (OR 1.7, 95% CI 1.7–1.8), congestive heart failure (OR 2.0, 95% CI 1.9–2.0) and renal failure (OR 1.7, 95% CI 1.6–1.8). Groups formed by combinations of these 3 factors had an observed 5-year risk of death varying between 22% and 91%.
INTERPRETATION: Among community-dwelling older adults with newly identified dementia in Ontario, the majority died or were admitted to a long-term care home within 5 years. This information may be helpful for discussions on prognosis and need for admission to long-term care.
Dementia refers to a group of symptoms resulting from neurodegeneration — including Alzheimer disease, vascular dementia, Lewy body dementia and frontotemporal dementia — that affects memory and brain function and interferes with everyday functioning.1 The global prevalence of dementia is increasing as the population ages2 and is expected to triple by 2050.3 Although there are exceptions, dementia is associated with a limited life expectancy.4 Because of the progressive course of cognitive decline, dementia is also a major contributing factor to the placement of individuals in long-term care homes (i.e., institutional long-term care facilities that provide 24-hour nursing and personal care), and in Canada’s largest province, Ontario, more than 70% of residents of long-term care homes have dementia.5
For people with dementia, their families and clinicians, understanding the likely disease trajectory is important for planning and making appropriate decisions about care. Many studies have focused on describing the risk factors for death for people living with dementia; among key predictors are older age, male sex, higher disease severity and dementia subtype.6–11 An important limitation of these studies, however, is their use of specific study cohorts (e.g., enrolment from specialized memory clinics) restricting their generalizability. In addition, competing disease trajectories and transitions of care, such as placement in a long-term care home, are typically not described.
Despite the high prevalence of dementia before death, clinicians often do not discuss mortality prognosis after a new diagnosis of dementia, which may be partly because of discomfort with discussing death and dying12 and exacerbated by a lack of resources to support those discussions. The utility of clinical prediction tools for other diseases (e.g., the Framingham Risk Score for heart disease) has been well documented.13 Although a similar prospective cohort of those with dementia does not exist, routinely collected health administrative data can be used at the very least to describe the experience after diagnosis of dementia. Such information may be helpful for clinicians to facilitate planning discussions on end-of-life care, including goals regarding care planning (e.g., do-not-resuscitate orders), home care and admission to a long-term care home.
This study examined the association between a newly documented diagnosis of dementia and the risk of admission to a long-term care home and death at 5 years. This information may be used by health care providers in their discussions about survival with patients with dementia and their families.
Methods
Study design
We conducted a population-based retrospective cohort study. Our study setting was Canada’s largest province, Ontario, where public health insurance covers the cost of many medically necessary health care services for more than 14 million residents. This includes all hospital and physician services, as well as some pharmaceutical, home care and long-term care home services. We captured newly documented diagnoses of dementia between Jan. 1, 2010, and Dec. 31, 2012, and followed these individuals over 5 years to look at our 2 primary outcomes of interest: death and admission to a long-term care home.
Data sources and definitions
Dementia cohort selection
We included all individuals aged 65 years and older, living in the community, whose dementia was first documented between Jan. 1, 2010, and Dec. 31, 2012. We excluded individuals who lost provincial health insurance eligibility during the 5-year period, were older than 105 years at the time of first documented diagnosis of dementia, had an invalid death date or for whom information on neighbourhood income quintile and rurality was missing (Appendix 1, Supplemental Table 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.190999/-/DC1).
We used linked health administrative databases to identify and characterize people with dementia. These data sets were linked using unique encoded identifiers and analyzed at ICES. ICES is an independent, nonprofit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyze health care and demographic data, without the need to obtain consent, for health system evaluation and improvement. Previous work has shown that these databases have high reliability and validity.14–21 These databases included the following (variables used are summarized in Appendix 1, Supplemental Table 2):
The Canadian Institute for Health Information (CIHI) hospital Discharge Abstract Database, which provided detailed diagnosis and treatment information during hospital admission;
The Ontario Health Insurance Plan physician claims database, which provided physician billing information;
The Ontario Drug Benefit program database, which provided information on all covered drug therapies dispensed to eligible individuals, including all Ontario residents aged 65 years or older;
The Resident Assessment Instrument–Home Care database, which provided details on individuals assessed for long-stay home care;
The Continuing Care Reporting System, which provided information on all long-stay residents of long-term care homes, using the validated Resident Assessment Instrument–Minimum Data Set (RAI-MDS); and
The Registered Persons Database, which provided information on age, sex and date of death.
We defined diagnosis of dementia as the first incidence of dementia documentation from health administrative data, using a previously validated algorithm for Alzheimer and related dementias, whose sensitivity is 79.3% and specificity is 99.1%.22 This algorithm identified a new diagnosis of dementia using any of 3 criteria: from the CIHI Discharge Abstract Database, a relevant International Statistical Classification of Diseases and Related Health Problems, 10th revision code recorded on hospital discharge; from the Ontario Health Insurance Plan, a relevant diagnostic billing code recorded on 3 physician billings separated by 30 days, within a 2-year period; or from the Ontario Drug Benefit program, the dispensing of any cholinesterase inhibitor. As a result of known underdiagnosis and documentation of dementia by physicians23,24 and moderate sensitivity from the validated algorithm used,22 we supplemented this definition by including individuals identified as having dementia through assessments for home care (Resident Assessment Instrument-Home Care) and long-term care (Continuing Care Reporting System).25 We considered the date on which dementia was first documented to be the index date.
Covariate and outcome definitions
We determined age and sex at the index date using the Registered Persons Database. Postal codes were linked to 2011 Statistics Canada census data26 at the index date to determine neighbourhood income quintile and residential setting. We identified primary care models through the Client Agency Program Enrolment registry to identify remuneration models for family physicians. These included fee-for-service models, which reimbursed physicians primarily on a per-visit fee schedule; capitation models, which reimbursed physicians primarily on age- and sex-based capitation rates for rostered patients; not rostered to a family physician; and other.
We identified the presence of complex chronic diseases among our cohort, using previously described methods.27 We defined organ failure as the presence of at least 1 of the following diseases: chronic obstructive pulmonary disease (COPD), congestive heart failure or renal failure. More details regarding ascertainment of the various chronic diseases are provided in Appendix 1, Supplemental Table 3.
We identified incident admissions to long-term care homes using a combination of physician billing codes and drug claims, as has been done in previous work.28 All-cause mortality was determined from the Registered Persons Database.
Statistical analysis
We examined the association between patient characteristics and our primary outcomes of admission to a long-term care home and death at 5 years. We performed survival analysis using Kaplan–Meier curves for death at 5 years, stratifying by covariates of interest. We used a log-rank test to test for differences between the strata. We then conducted multivariable logistic regression modelling for death at 5 years, including available covariates previously identified in the literature to be associated with death in individuals with dementia. Covariates included were age, sex, income quintile, residential setting, primary care model (based on expert consensus, from clinicians and epidemiologists familiar with the data, cohort, and clinical context) and chronic conditions (cancer, COPD, congestive heart failure, renal failure, stroke and diabetes mellitus). The model included chronic conditions that were highly prevalent in the cohort and were likely to affect death risk, based on other studies.29–34 We performed cumulative incidence analysis of admissions to a long-term care home at 5 years, accounting for competing risk of death, stratified by covariates of interest. We used Gray’s test to examine for differences between the strata. All statistical tests were 2-tailed and we used p < 0.05 to determine statistical significance. We used SAS 9.4 (SAS Institute Inc., Cary, NC) for all analyses.
We developed a visual representation of findings of death risk at 5 years (from ICES data). We selected covariates with the largest odds ratio (OR) in the multivariable model, namely sex, age and presence of any 1 of 3 conditions (COPD, congestive heart failure and renal failure) that were grouped as organ failure. We derived 5-year death rates from descriptive, population-level data. We calculated the values presented by taking the crude number of individuals who died in the 5 years after diagnosis in a specified risk category divided by the total number of individuals in that category. Secondarily, we also present similar supplemental visuals for median survival and median time to admission to a long-term care home.
Ethics approval
ICES is a prescribed entity under section 45 of Ontario’s Personal Health Information Protection Act. Section 45 authorizes ICES to collect personal health information, without need to obtain consent, for the purpose of analysis or compiling statistical information with respect to the management of, evaluation or monitoring of the allocation of resources to or planning for all or part of the health system. Projects conducted under section 45, by definition, do not require review by a research ethics board. This project was conducted under section 45, and approved by ICES’ Privacy and Legal Office.
Results
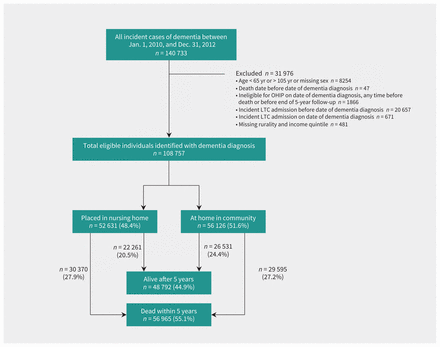
The final community-dwelling cohort included 108 757 individuals aged 65 and older, whose initial documentation of dementia occurred between Jan. 1, 2010, and Dec. 31, 2012 (Figure 1). The cohort had an average age of 82.0 years and contained more women than men (Table 1).
Flow diagram of patients identified in the study. Note: LTC = long-term care, OHIP = Ontario Health Insurance Plan.
Characteristics of the cohort of community-dwelling older adults (≥ 65 yr) at diagnosis of dementia, stratified by death and admission to long-term care home at 5 years follow-up*
Table 1 compares those who were still alive versus those who died over the 5 years of follow-up. Individuals who died were older (mean age 83.9 v. 79.8 yr when they entered the cohort). Being male, having 5 or more chronic conditions, and particularly organ failure, were also associated with death. Table 1 also compares older adults who remained in the community versus those who were admitted to a long-term care home within 5 years. Individuals placed in long-term care homes were older (mean age 82.8 v. 81.3 yr); women and those in rural regions were more likely to be admitted to a long-term care home than their counterparts.
Among those identified with dementia, 48.4% had been admitted to a long-term care home at some point during the 5-year period (Figure 1). At the end of 5 years, 55.1% of the cohort had died; more than half of these individuals were admitted to a long-term care home before death. Among those identified with dementia, only 1 in 4 (24.4%) were still alive and living in the community after 5 years.
Predictors of death at 5 years
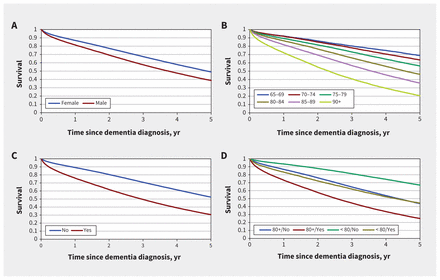
Figure 2 shows 5-year Kaplan–Meier survival curves after diagnosis of dementia. The median survival time for the full cohort was 4.4 years. The median survival time for men was 3.7 years and 4.9 years for women. For individuals diagnosed with dementia at younger than 80 years, the median survival was > 5.0 years, while those diagnosed with dementia at ages 80–84 years had a median survival of 4.6 years; 85–89, 3.6 years; and 90 or older, 2.3 years. Individuals with organ failure had a median survival time of only 3.0 years. The combination of older age and organ failure was particularly poor: individuals aged 80 years or older with organ failure had a median survival time of only 2.5 years.
Survival over the 5-year follow-up after diagnosis of dementia. Note: Kaplan–Meier survival curves for death, stratified by (A) sex (male or female), (B) age group (65–69, 70–74, 75–79, 80–84, 85–89, ≥ 90 yr), (C) organ failure (presence or absence), and (D) age and organ failure (≥ 80 yr with no organ failure, ≥ 80 yr with organ failure, < 80 yr with no organ failure, < 80 yr with organ failure).
The results of the multivariable logistic regression to determine risk factors for death at 5 years after diagnosis of dementia are shown in Table 2. Age was by far the most significant risk factor: the odds ratio for an individual diagnosed with dementia at 90 years or older as compared with age 65–69 years was 9.5 (95% confidence interval [CI] 8.8–10.2). Compared with women, being male was also associated with greater odds of death (OR 1.7, 95% CI 1.6–1.7). There was a small but statistically significant lower odds of death for individuals living in a wealthier neighbourhood (when comparing the top 2 income quintiles to the lowest quintile), and urban location compared with a rural location. Congestive heart failure had the largest odds ratio of the chronic diseases examined (OR 2.0, 95% CI 1.9–2.0). Although we did not aim to build a prediction model, the discrimination of the model was good, with a concordance statistic of 0.72.
Multivariable logistic regression for death at 5 years after diagnosis of dementia
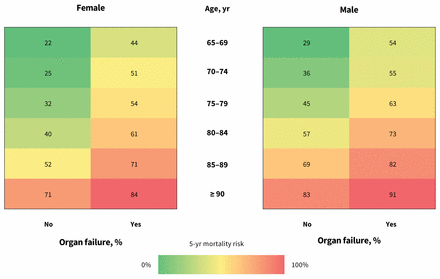
Visual representation of death risk
Figure 3 shows death risk based on the most significant predictors: older age, male sex and presence of organ failure. Risk of death varied considerably, between 22% for women aged 65–69 years without organ failure and 91% for men aged 90 years or older with organ failure. Supplemental Figure 1 in Appendix 2 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.190999/-/DC1) shows a visual representation of median survival and survival at the 25th and 75th percentiles.
Visual representation of 5-year risk of death for community-dwelling older adults given a new diagnosis of dementia. Note: Percentages represent the crude, unadjusted proportions in each subgroup who had died by the end of the 5-year follow-up period. These were not built using prediction models, but rather display the observed outcome of individuals within each subgroup in our cohort. Organ failure was defined as at least 1 of chronic obstructive pulmonary disease, congestive heart failure or renal failure.
Transition to placement in a long-term care home
We used cumulative incidence curves to depict admission to a long-term care home after diagnosis of dementia (Appendix 2, Supplemental Figure 2). Women were at higher risk of being admitted to long-term care homes than men. The risk of placement in a long-term care home increased significantly as age increased. Individuals with lower income (bottom 3 quintiles) were also more likely to transition to long-term care homes. Those with organ failure initially had higher risk of being admitted to a long-term care home, but eventually ended up with lower risk at the end of 5 years. Supplemental Figure 3 in Appendix 2 shows a visual representation of the median cumulative incidence function for admission to a long-term care home within 5 years, along with the 25th and 75th percentiles.
Interpretation
Among our population-based cohort of community-dwelling older adults given a new diagnosis of dementia, 55.1% died and 48.4% were placed in a long-term care home at some point over the 5-year follow-up period. At the end of 5 years, only 1 in 4 individuals were alive and still living in the community.
Our study confirms that dementia is a life-limiting illness. Indeed, it has poorer 5-year survival from initial diagnosis than common cancers in the Canadian population, including prostate (95% 5-yr survival), breast (87%) and colorectal cancers (64%).35 Dementia is often overwhelming for caregivers because of physical, emotional and economic stresses related to a person’s decline; information about prognosis can be helpful to alleviate concerns stemming from uncertainty. However, discussions about advanced care planning — which should incorporate the high likelihood for admission to a long-term care home and death — have several barriers.36 Studies consistently find that individuals with dementia are far less likely to receive palliative care than individuals with other diseases,37–40 which reflects suboptimal end-of-life care for patients and may lead to families being unprepared for their loved one’s death.41
Our approach to displaying death rate visually (Figure 3) may be useful for informing conversations between health care providers and persons with dementia. Three key characteristics contribute to our model — age, sex and presence of organ failure — and shows the observed 5-year death rate, which varies between 22% and 91%. Such a depiction may help patients and caregivers develop a clearer understanding of the implications after a diagnosis of dementia and make care decisions that are better informed. Although other visual representations for estimating death after a new diagnosis of dementia exist, these can be cumbersome to calculate, with more than a dozen variables required; in addition, they predict survival to only 12 months, a time horizon that encourages short-term decision-making only.10,42
Similar to other studies, the risk of death among our cohort increased with older age and male sex.6,9,11 Moreover, we found that the presence of certain chronic conditions accelerates death, including COPD, congestive heart failure and renal failure. Although those dying with dementia are thought to follow a “frailty” trajectory, 43 the crossover of organ failure and dementia specifically affects prognosis — an observation not previously well studied. Interestingly, we found the crossover of cancer and dementia to be weaker with respect to the rate of death and admission to a long-term care home over 5 years; more studies are needed to elucidate the mechanisms underlying these associations.
Our study has several key strengths. This large population-based cohort allows for better generalizability than previous studies of specific groups, such as individuals from specialized memory clinics. We used new dementia documentation to minimize the risk of length bias, and we were able to capture an important transition among this cohort — admission to a long-term care home — alongside survival.
Limitations
There are several important limitations of this study. First, we restricted our cohort to community-dwelling older adults at diagnosis, and so are unable to describe whether younger individuals, or older adults diagnosed with dementia in long-term care homes or other institutions, follow a similar trajectory. The databases we used also do not capture many First Nations individuals who access health care services through federal funding sources.
Second, it is difficult to establish a precise diagnosis date given that the onset of dementia usually occurs insidiously over a long period of time. However, our index date is the first time a hospital admission code, physician claim code or a dementia-specific prescription was filled, which would estimate clinically when dementia was substantially affecting an individual’s function.
Third, the visual representation of death risk we developed discriminates risk of death based on 3 pieces of information; however, the focus of this study was not on predictive algorithm development, and model performance was therefore likely sacrificed for parsimony.
Fourth, the algorithm we used to identify the cohort is unable to determine the severity of dementia or to differentiate between the different subtypes.22 As such, we are unable to report on whether these have different rates of death and admission to a long-term care home.
Finally, the generalizability of our findings as they relate to admission to a long-term care home is likely strong for high-income countries similar to Canada, but may be reduced in developing countries. Placement in a long-term care home, however, can be understood as a proxy for an individual with dementia being unable to live independently and safely without substantial support, so this information may be helpful for patients, families and clinicians.
Conclusion
Among community-dwelling older adults with newly identified dementia in Ontario, Canada, 55.1% died and 48.4% were admitted to a long-term care home within 5 years, and only 24.4% were both alive and living in the community. Although those dying with dementia have traditionally been thought to follow a “frailty” trajectory, we showed that the presence of organ failure significantly affects prognosis. We found that 5-year death risk was concentrated in 3 factors: age, sex and organ failure. We present death risk based on these 3 factors, which can be used by clinicians to open a discussion with patients and their families regarding risk of death and admission to a long-term care home. To minimize the societal and economic burden of dementia, further research is needed to better understand how to best support individuals with dementia and their caregivers to live safely and independently in the community.
Acknowledgement
The authors thank IMS Brogan Inc. for use of its Drug Information Database, made available through the Ontario Drug Benefit program.
Footnotes
Competing interests: Sarah Spruin reports receiving a grant from the Canadian Institutes of Health Research, during the conduct of the study. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Gregory Huyer and Peter Tanuseputro conceived the study. Gregory Huyer, Sarah Spruin, Amy Hsu, Stacey Fisher, Douglas Manuel, Susan Bronskill, Danial Qureshi and Peter Tanuseputro designed the study. Sarah Spruin, Gregory Huyer and Catherine Brown analyzed the data. Gregory Huyer, Catherine Brown and Peter Tanuseputro drafted the manuscript. All of the authors revised the manuscript critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This study was funded by a grant from the Canadian Institutes of Health Research (MOP-142237) and the Bruyere Centre for Individualized Health.
Data sharing: The data set from this study is held securely in coded form at ICES. While data sharing agreements prohibit ICES from making the data set publicly available, access may be granted to those who meet pre-specified criteria for confidential access, available at www.ices.on.ca/DAS. The full data set creation plan and underlying analytic code are available from the authors upon request, understanding that the computer programs may rely upon coding templates or macros that are unique to ICES and are therefore either inaccessible or may require modification.
Disclaimer: This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. The funders had no influence on the contents of this paper. Parts of this material are based on data and/or information compiled and provided by the Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions and statements expressed in the material are those of the author(s), and not necessarily those of CIHI.
- Accepted February 13, 2020.