First-person consent for organ donation after medical assistance in dying (MAiD) or withdrawal of life-sustaining measures (WLSM) should be an option in jurisdictions that allow MAiD or WLSM and donation after circulatory determination of death.
The most important ethical concern — that the decision for MAiD or WLSM is being driven by a desire to donate organs — should be managed by ensuring that any discussion about organ donation takes place only after the decision for MAiD or WLSM is made.
If indications for MAiD change, this guidance for policies and the practice of organ donation after MAiD should be reviewed to ensure that the changes have not created new ethical or practical concerns.
In Canada, organ donation from deceased donors is a common practice that saves or improves the lives of more than nearly 2000 Canadians every year, accounting for more than 3 of 4 of all transplanted organs.1 Deceased donation is permitted after either neurologic or circulatory determination of death, with the latter accounting for 25% of all organs donated in Canada in 2017.1 The current Canadian guideline recommendations for donation after circulatory determination of death, published in 2006, address the conventional scenario of an unconscious, incapable, critically ill patient not expected to survive the withdrawal of life-sustaining measures (WLSM).2
Two recent developments in Canada have led to a scenario not anticipated at the time of the 2006 guideline — requests for donation after circulatory determination of death from patients who are conscious and capable. The first development is the legalization of medical assistance in dying (MAiD),3–5 and the second, an anecdotal increase in requests for organ donation by patients with advanced neuromuscular diseases who have decided to have life-sustaining measures withdrawn. The ability of donors to give first-person consent for both MAiD or WLSM and organ donation creates emotional and moral challenges for health care professionals, and raises unprecedented ethical and practical challenges for patients, families, health care professionals and institutions, and society.
Prompted by requests from patients, Canadian practitioners requested guidance for policy development. In response, Canadian Blood Services worked in consultation with the Canadian Neurological Sciences Federation and in collaboration with the Canadian Critical Care Society, the Canadian Society of Transplantation and the Canadian Association of Critical Care Nurses to develop ethical, legal and clinical guidance for policies about managing deceased organ donation in conscious, competent donors. The full guidance document is available in Appendix 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.181648/-/DC1. Here, we present key considerations and selected recommendations from the full guidance document.
Scope
The target audience of this guidance consists of clinicians, organ donation organizations, end-of-life care experts, MAiD providers and policy-makers. This document is intended to inform policies related to offering organ and tissue donation to patients who have made a decision that will lead to imminent death. These are conscious, competent patients who have chosen to withdraw mechanical ventilation, including invasive or noninvasive mechanical ventilation; conscious, competent patients who have chosen to withdraw extracorporeal support, including extracorporeal membrane oxygenation or other mechanical circulatory support; and eligible patients who have requested MAiD.
The issues that are out of scope for this guidance include the ethics of MAiD or WLSM, best practices for MAiD or WLSM independent of organ and tissue donation, donation by euthanasia (i.e., organ donation that does not adhere to the dead donor rule) and living organ donation.
Methods
Canadian Blood Services organized and funded the development of this guidance in response to requests from practitioners and policy-makers.
Guidance panel
Canadian Blood Services convened a panel of experts from relevant fields, including organ donation, ethics, law, intensive care, end-of-life care and neurology (S.S., M-C.F., D.B., C.S., A.G., D.Y. and V.G.). Members of the Canadian Blood Services Deceased Donation team also participated (C.G., A.A., J.L.). The panel was cochaired by J.D. and M.S.
Development process
Canadian Blood Services hosted a guidance development meeting in Toronto on May 15 and 16, 2017. Medical, legal and ethical experts; patients considering MAiD and organ donation; and an expert from Belgium participated (see full participant list in Appendix 1).
The objectives were to analyze organ and tissue donation in the conscious, competent patient from legal, medical and ethical perspectives; develop and disseminate guidance for policies and clinical procedures for offering organ and tissue donation to patients who have made a decision that will lead to imminent death (see Scope section); develop a knowledge translation strategy that includes all relevant stakeholders; and identify questions for research.
The guidance panel defined the scope, assumptions and key considerations of the guidance document and the workshop a priori (Appendix 1), based on the requests for guidance received by Canadian Blood Services. The guidance was developed via a process previously used for the national recommendations on organ donation after cardiocirculatory death.2
Before the workshop, the guideline panel commissioned a public opinion survey from Ipsos-Reid. Panel members searched for published studies and guidelines relevant to important topics, such as previous experience with organ donation in the conscious, competent adult (V.G., D.Y. and A.G.), patients with amyotrophic lateral sclerosis as organ donors (C.S.) and conscientious objection (D.B., V.G.), and developed background documents to guide and support discussion (Appendix 1). We provided these documents to all participants in advance of the workshop.
We structured the workshop around plenary presentations by Canadian and international clinicians, organ donation and transplantation ethicists and legal experts, a coroner and patient partners. The workshop included participants with a range of opinions about the practice of MAiD, and participants were free to share those opinions, but regardless of their support or opposition to MAiD, participants thought that if MAiD and organ donation were legal options, it was important to develop guidance to inform policies about them.
Participants were divided into smaller groups throughout the meeting to discuss and make recommendations regarding specific challenge questions that were informed by the background documents and expert presentations. Key points and conclusions from these groups were shared and discussed in plenary. Based on the material and notes from the small group and plenary discussions, the guidance panel developed and refined a series of recommendations meant to reflect the consensus of participants at the workshop. The guidance panel members agreed unanimously on the final guidance document presented here.
Given that this guidance was intended to address ethical and practical considerations relevant to policies and practice, the guidance panel did not think it was appropriate to assign a relative strength to any recommendation or rate the quality of evidence (similar to the previous guidance on donation after circulatory determination of death2). Some practical recommendations were informed by limited clinical experience and some unpublished data, but were not evidence based.
Management of competing interests
All members of the guidance panel declared any competing interests before any presentations to panel members while planning the meeting, and all presenters at the meeting declared their competing interests before their presentations.
To manage competing interests further, the scope of the guidance did not include a reference to any medications or devices, or any technical aspect of MAiD or donation after circulatory determination of death. Panel members, presenters and meeting participants were free to declare their personal views on MAiD or donation after circulatory determination of death but were not required to do so. All participants were from jurisdictions where MAiD and donation after circulatory determination of death are legal and practised.
Recommendations
Selected recommendations and key considerations are presented below. See Table 1 for a summary of all recommendations. More information on the evidence from the scoping reviews and discussion supporting each recommendation can be found in the full guidance document (Appendix 1).
Summary of recommendations
Deceased organ donation in conscious and competent patients
Medically suitable, conscious and competent patients who provide first-person consent to end-of-life procedures should be given the opportunity to donate organs and tissues. Patients who seek MAiD or WLSM should not be prohibited from donating organs and tissue.
Before consenting to WLSM or MAiD, patients should carefully consider all end-of-life options with their physician or health care professional.
Withdrawal of life-sustaining measures, MAiD and organ donation enjoy broad public support, both individually and in combination, and there is an existing legal and ethical framework for each in Canada (Appendix 1). Workshop participants identified potential problems that might arise when combining these practices (e.g., that the decision to donate organs might influence the decision to pursue MAiD), but these were felt to be manageable. Organ donation has taken place after WLSM and MAiD in a relatively small number of cases in Canada and other jurisdictions6–8 without any apparent negative effects.
Conversations about donation
The decision to proceed with MAiD or WLSM must be separate from, and must precede, the decision to donate.
All eligible, medically suitable patients should be given an opportunity to consider organ and tissue donation, consistent with provincial or territorial required referral legislation, regional policy and ethical principles of respect for autonomy and self-determination. However, this must be reconciled with regional values and health care culture. Initially, some jurisdictions might prefer to begin with systems that respond only to patient-initiated requests.
The most important ethical concern that arises from the combination of MAiD or WLSM and organ donation is the possibility that the decision to proceed with MAiD or WLSM might be driven by the desire to donate organs.7 Workshop participants felt that the best way to mitigate this risk is to ensure that organ donation discussions do not occur until after the decision is made regarding MAiD or WLSM. An additional safeguard would be to not offer organ donation in cases of MAiD or WLSM specifically, but respond only to organ donation requests initiated by patients themselves. However, participants were concerned that this could undermine a patient’s autonomous right to donate their organs.
Consent
The patient must have the ability to provide first-person consent to MAiD or WLSM as well as to organ and tissue donation.
The individual should be informed and understand that they may withdraw consent for MAiD or donation at any time, and that withdrawal of consent for donation does not affect their consent for, or access to, MAiD or WLSM.
Although there are potential concerns with first-person consent for organ donation after circulatory determination of death (including the permissibility of treatments administered to the dying donor solely for the health of the donated organ),9 the informed, contemporaneous consent of a conscious and competent patient is the gold standard for decision-making in medicine. 10 Family concerns are important, and families must be supported throughout the donation, both for their own sake and to retain trust in the organ donation process. However, family refusals should not override persistent requests from capable patients to donate organs.11
Donor testing and evaluation
Primary care physicians, staff of organ donation organizations, MAiD providers and transplant teams should work to minimize the impact and inconvenience to the patient of donating their organs. This could include scheduling home visits for blood draws and coordinating investigations (e.g., x-rays, ultrasound) to minimize hospital visits and inconvenience to the individual.
Anecdotal experience from early cases suggested that the burdens of deceased organ donor testing may be substantial for patients on an outpatient basis, particularly when they have grievous and irremediable conditions and a limited life expectancy. Outpatient assessment of cardiac and pulmonary function should be reduced as much as possible to avoid losing donors, and home care should be engaged when appropriate. In some cases, donors may opt to donate fewer organs to limit the burden of testing.
Determination of death
The dead donor rule must always be respected. Vital organs can be procured only from a donor who is already deceased; the act of procurement cannot be the immediate cause of death.
For determination of death, absence of a palpable pulse alone is not sufficient. If arterial monitoring is not available, alternate means of determining absence of anterograde circulation should be used in conjunction with absence of a palpable pulse, such as a carotid perfusion ultrasound, Doppler monitoring, aortic valve ultrasound or an isoelectric electrocardiogram to determine asystole.
As with all cases of donation after circulatory determination of death, death should be confirmed by a second physician after a 5-minute “no touch” period of continuous observation, during which time no donor-based interventions are permitted.
Donors must be deceased according to accepted criteria before organ retrieval.2 Medical assistance in dying by vital organ donation is not an acceptable mechanism of MAiD.12 Because the usual invasive monitoring is not present during MAiD, a noninvasive approach may be needed to confirm circulatory death.13 The organ procurement process cannot be implicated in the death of the patient in any way.
Protection for patients
Separation of decisions
To avoid any real or perceived conflict of interest, health care practitioners should separate the decision regarding WLSM or MAiD from discussions concerning donation. Providers who are assessing eligibility for MAiD should not be involved in donation discussions.
The primary health care team should acknowledge patient inquiries concerning donation that are made before a decision to proceed with MAiD or WLSM. General information on deceased organ and tissue donation may be provided. However, specific discussion and decisions pertaining to donation should wait until the decision to proceed with MAiD or WLSM has been finalized.
Separation of roles
Consistent with current guidelines and practice regarding donation after circulatory determination of death, separation should be maintained between the end-of-life care, donation and transplant teams. Surgical recovery and transplant teams should not be involved in the patient’s end-of-life care or MAiD or WLSM procedure. The only exception is insofar as they may provide guidance for minimal requirements for donor investigations or premortem interventions.
That an organ donor received MAiD should not be disclosed to the potential recipient during allocation; however, medically relevant information regarding their underlying disease may be disclosed according to guidelines for exceptional distribution, where applicable.
Transplant teams should be separate from those providing end-of-life care to the donor, to ensure there are no competing interests in terms of providing comfort care and ensuring the suitability of the organs for transplant.2 A guideline on WLSM is available and its recommendations can be applied regardless of whether the person is a potential organ donor or not.14
The circumstances of the donor’s death should not be disclosed to the recipient in cases of MAiD or WLSM or any other donation situation.15 The donor’s underlying medical condition may be relevant to the recipient and their transplant team, and can be disclosed under existing recommendations for exceptional distribution.16
Amyotrophic lateral sclerosis and neurodegenerative diseases
People with amyotrophic lateral sclerosis and patients with other nontransmissible neurodegenerative diseases should be offered the opportunity to donate organs after their death.
Organ donation organizations should exercise caution regarding allocation of organs from donors with undiagnosed or rapidly progressive neurodegenerative diseases, as these may pose elevated risks to recipients. Organ allocation in this context should follow existing exceptional distribution policies and practices.
All cases of amyotrophic lateral sclerosis or other neurodegenerative diseases that arise in transplant recipients should be reported to Health Canada, to determine potential associations with donor illness and the baseline risk of neurodegenerative illness in transplant recipients (e.g., whether transplant recipients, in general, have rates of amyotrophic lateral sclerosis that differ from the general population).
Physicians who follow organ recipients should be aware that the donation was by a patient with neurodegenerative disease such as amyotrophic lateral sclerosis, aware of theoretical transmission risk of neurodegenerative diseases, and cognizant of symptoms or complaints that warrant further investigation by a neurologist to determine if a neurodegenerative disease is present.
Many organ donation organizations do not accept organs from people with amyotrophic lateral sclerosis or other undiagnosed neurodegenerative conditions. Some authors have urged caution in this area,17 but people with amyotrophic lateral sclerosis have successfully donated organs after WLSM,18 and there appears to be no elevated risk of developing amyotrophic lateral sclerosis or many other neurodegenerative conditions after donation from selected persons with amyotrophic lateral sclerosis (Appendix 1). Because data are scarce about many neurodegenerative conditions, ongoing follow-up with the recipient is needed.
Decisions to use organs from donors with undiagnosed or rapidly progressive neurodegenerative conditions should be individualized based on risk and benefit because of the concern about transmissibility. Some organ transplants are primarily for improving quality of life or independence; others are clearly for survival.
Conscientious objection
Health care professionals may exercise a conscientious objection to MAiD or WLSM specifically, but they should strive to accommodate the wishes of the donor by ensuring that their objection to MAiD or WLSM does not impede the ability of the patient to donate.
Although most regulatory bodies in Canada have policies that govern conscientious objection,19 it is not clear how those policies apply to organ donation (see detailed explanation in Appendix 1). Conscientious objectors may not wish to participate in a process that facilitates MAiD or WLSM. But once the donor is deceased, it is difficult to apply the same framework to organ retrieval, transport or transplantation, as none of these acts facilitate MAiD or WLSM. Objectors may feel that participation in retrieval, transport or transplantation is a type of validation of how the organs became available. However, it is unclear why this logic would not apply to donors who died from suicide or homicide. Inconsistent application of conscientious objection policies may lead to inequitable allocation of organs20 or place substantial burdens on some staff and institutions.21
Implementation
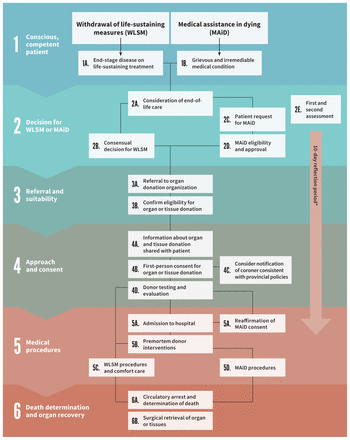
Offering the opportunity for conscious, competent patients to donate their organs after their death by WLSM or MAiD will affect institutions, health care professionals and society. An overview of the process is provided in Figure 1, but this practice will require mechanisms for oversight, data collection and reporting. Research on quality assurance and improvement to ensure this option for care is performed ethically and safely is also important.
Clinical pathway for organ donation in conscious, competent patients in Canada. Note: MAiD = medical assistance in dying, WLSM = withdrawal of life-sustaining measures. *The 10-day reflection period begins from the day that the patient signs their written request, which should be after the first assessment of eligibility. This reflection period can be shortened if both assessors agree that the patient appears likely to die or lose capacity.
Medical assistance in dying remains a controversial practice for many, and those who do not accept the practice of MAiD may not be able to apply this guidance. There are existing reporting mechanisms for MAiD3,4,22,23 and organ and tissue donation in all jurisdictions, but none for WLSM. Documentation and reporting mechanisms will need to be modified to ensure that key recommendations were followed, such as the requirement for the decision on MAiD or WLSM to precede the decision on donation, or the necessity for a registry to track the development of neurodegenerative disease in recipients.
There is no specific timeline to update this guidance for policy, but any changes to the eligibility criteria for MAiD (e.g., advance consent for MAiD for incapable patients) or the practice of donation after circulatory determination of death would require a review of this document to ensure that it covers any new ethical or practical concerns.
Other guidelines
There are no previous published guidelines for policies and the practice of deceased organ and tissue donation for conscious, competent donors with respect to both MAiD and WLSM. There is a Dutch guideline for the practice of organ donation after euthanasia (but not WLSM),24 and manuals have been published in Canada25 and the Netherlands26 to help health care organizations develop a program for organ donation after MAiD. There are also Canadian guidelines for WLSM14 and donation after circulatory determination of death,2 but the latter does not assume that first-person consent is an option. There are no published guidelines regarding the advisability of organ donation from donors with neurodegenerative diseases such as amyotrophic lateral sclerosis, or the role of conscientious objection for organ donation in this context, and research is needed to inform practice in this area, as indicated in the present report.
Gaps in knowledge
Notably, directed deceased donation in conscious, competent patients is not recommended, although directed donation is permitted for living donors in many jurisdictions under specific circumstances. 27 If a person was eligible for MAiD and had a relative or friend who was waiting for an organ, it would be difficult to exclude the possibility that the decision regarding organ donation had driven the request for MAiD, either to facilitate the donation process or to enhance the function of the transplanted organ.
A recent report of 2 Dutch cases of organ donation after MAiD initiated in the home28 raises the possibility of that practice occurring in Canada. Allowing the MAiD procedure to start outside the hospital would be more patient centred, and possibly improve the patient and family experience, but there are practical considerations that are not covered in this guidance (e.g., the logistics of transportation to hospital mid-procedure).
Finally, although some authors have suggested that organ donation after MAiD might have a substantial impact on organ availability, 29,30 the practice of organ donation after MAiD in Canada is still rare. More data about this practice must be collected to determine whether this guidance document is helpful or needs to be updated.
Conclusion
In this report, we present guidance for policy and the practice of deceased organ donation in the conscious and competent donor. Given the relatively low incidence of MAiD and WLSM among people who are eligible to donate organs in Canada, this practice is unlikely to substantially affect the supply of organs for potential recipients on waiting lists. The purpose of this guidance is to inform the development of policies to help clinicians navigate the medical, legal and ethical challenges that arise when they respect a person’s autonomous right to request MAiD or WLSM and organ donation.
Although many of these recommendations were informed by experience and data, areas of research remain, to ensure that a person’s final wishes are honoured without placing their family, health care providers or potential recipients at risk of harm.
Acknowledgements
This guidance was developed on behalf of Canadian Blood Services in collaboration with the Canadian Critical Care Society, the Canadian Society of Transplantation, and the Canadian Association of Critical Care Nurses. This report is dedicated to Dr. Shelly Sarwal and Dr. Linda Panaro, who provided unique perspectives and thoughtful insights into the development of this guidance. Their knowledge and advocacy informed and guided the workshop process and has helped in the creation of this document, which will guide practice and inform policy development at the provincial and territorial and national level. The planning committee would also like to acknowledge all individuals who contemplate organ donation as part of their end-of-life care after making the decision for medical assistance in dying or withdrawal of life-sustaining measures.
Footnotes
CMAJ Podcasts: author interview at https://soundcloud.com/cmajpodcasts/181648-guide
Competing interests: Canadian Blood Services reports receiving grants from Health Canada and all provincial and territorial governments, during the conduct of the study. James Downar reports receiving personal fees from Boehringer-Ingelheim (Canada), Novartis and the Ontario College of Family Physicians, outside the submitted work. Dr. Downar is also the previous co-chair of the Physicians’ Advisory Committee for Dying with Dignity Canada, an advocacy group for the legalization of medical assistance in dying in Canada. Sam Shemie reports receiving personal fees from Canadian Blood Services, during the conduct of the study. Vanessa Gruben reports receiving a grant from Canadian Blood Services, during the conduct of the study. Aviva Goldberg reports that as a nephrologist, she is primarily involved in the care of transplant recipients. No other competing interests were declared.
This article has been peer reviewed.
Guidance panel members: James Downar, Sam D. Shemie, Clay Gillrie, Marie-Chantal Fortin, Amber Appleby, Daniel Z. Buchman, Christen Shoesmith, Aviva Goldberg, Vanessa Gruben, Jehan Lalani, Dirk Ysebaert, Lindsay Wilson, Michael D. Sharpe.
Contributors: James Downar, Sam Shemie, Clay Gillrie, Amber Appleby, Jehan Lalani and Michael Sharpe conceived the guidance development project. All authors contributed to the design and conduct of the guidance development meeting, and the acquisition, analysis and interpretation of data. All authors drafted the manuscript, revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: The guidance development process was funded by Canadian Blood Services.












Podcast