Abstract
BACKGROUND: Emerging links between household cleaning products and childhood overweight may involve the gut microbiome. We determined mediating effects of infant gut microbiota on associations between home use of cleaning products and future overweight.
METHODS: From the Canadian Healthy Infant Longitudinal Development (CHILD) birth cohort, we tested associations between maternal report of cleaning product use and overweight at age 3, and whether associations were mediated by microbial profiles of fecal samples in 3- to 4-month-old infants.
RESULTS: Among 757 infants, the abundance of specific gut microbiota was associated with household cleaning with disinfectants and eco-friendly products in a dose-dependent manner. With more frequent use of disinfectants, Lachnospiraceae increasingly became more abundant (highest v. lowest quintile of use: adjusted odds ratio [AOR] 1.93, 95% confidence interval [CI] 1.08 to 3.45) while genus Haemophilus declined in abundance (highest v. lowest quintile of use: AOR 0.36, 95% CI 0.20 to 0.65). Enterobacteriaceae were successively depleted with greater use of eco-friendly products (AOR 0.45, 95% CI 0.27 to 0.74). Lachnospiraceae abundance significantly mediated associations of the top 30th centile of household disinfectant use with higher body mass index (BMI) z score (p = 0.02) and with increased odds of overweight or obesity (p = 0.04) at age 3. Use of eco-friendly products was associated with decreased odds of overweight or obesity independently of Enterobacteriaceae abundance (AOR 0.44, 95% CI 0.22 to 0.86), with no significant mediation (p = 0.2).
INTERPRETATION: Exposure to household disinfectants was associated with higher BMI at age 3, mediated by gut microbial composition at age 3–4 months. Although child overweight was less common in households that cleaned with eco-friendly products, the lack of mediation by infant gut microbiota suggests another pathway for this association.
Greater emphasis on cleanliness has led to widening use of disinfectants and other cleaning agents in the home.1 Household cleaning products have been associated with elevated risk of wheeze in persons using these products2 and in their children.3 The literature on risk for overweight is more limited and informed by national surveys of ingredients previously common in cleaning products, such as triclosan.4 According to a new study, high urinary levels of triclosan are evident in US adolescents with greater adiposity.5
Although not shown in trials of “usual” home use of cleaning products,6,7 microbial levels on household surfaces are effectively reduced when the daily dose of disinfectant is standardized.8 In fact, piglets born in an indoor environment, and then raised under conditions of continuous aerosolization with a disinfectant, have shown perturbed gut microbial composition compared with piglets not reared under these conditions.9 Indeed, concerns over the potential for antibacterial products to be too effective or even toxic has motivated use of “green” or eco-friendly alternatives.10,11 When tested, commercial or homemade eco-friendly products have efficacy comparable with bleach12,13 and other household disinfectants14 against some microbes but not others.
Central to the hygiene hypothesis of allergic disease is the microbial environment in which we live.15 According to Hesselmar and colleagues, protection from allergic disease may also involve microbes left on eating utensils after handwashing versus dishwasher use.16 Environmental microbes may also protect against metabolic disease, as evident from reports of reduced overweight in kindergarten children previously attending daycare.17 Because infants spend more than 80% of their time indoors,18 the home microbial environment is especially relevant to the maturation of their gut microbial ecosystem. Direct and indirect evidence of altered gut microbial colonization during infancy has been linked to allergic disease and overweight.19,20 Short-chain fatty acid metabolites produced by gut microbiota are involved in appetite regulation and in lipid and glucose metabolism; their heightened production is thought to play a role in development of overweight.21
Similar to other cohorts,22 the Canadian Healthy Infant Longitudinal Development (CHILD) study birth cohort was designed to assess the health impact of indoor environmental exposures, including household cleaning products. To date, a detailed evaluation of the impact of household cleaning agents on the gut microbiota of infants or on child overweight is lacking. Herein, we tested associations between household cleaning product use and infant gut microbial composition at 3–4 months of age in the CHILD cohort. Thereafter, we determined the association between cleaning product use and child overweight at age 3 and whether it was mediated by the gut microbial changes observed.
Methods
Study design
The present study includes a subsample of children from the CHILD population-based birth cohort (Figure 1). We recruited women during the second or third trimester of their pregnancy, and enrolled them in the study cohort if their newborns were a singleton live birth at ≥ 35 weeks of gestation with a birth weight of ≥ 2500 g. We excluded in vitro fertilization births because they were more likely to result in multiple gestations or preterm delivery (< 35 wk), which were study exclusion criteria. We excluded home births owing to lack of data on maternal intrapartum antibiotic prophylaxis. We followed the enrolled women throughout pregnancy and their children from birth to age 3 years.
Flowchart of the infants eligible for the study. CHILD = Canadian Healthy Infant Longitudinal Development.
Exposures
At 3–4 months postpartum, mothers completed questionnaires on aspects of their health, home environment and personal use of cleaning products (Appendix 1a, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.170809/-/DC1). They were asked: “Which of the following products [a list of 31 chemical-based products] do you personally use?” Available responses were daily, weekly, monthly, less than monthly and not used at all. From the 31 queried categories, we grouped cleaning products according to the mechanism of action, namely disinfectant, detergent and other (Appendix 1b). The frequency of use for each queried product was converted into 5 numeric scores: 0 for not used at all, 1 for less than once a month, 2 for monthly, 3 for weekly and 4 for daily usage. We summed the scores to produce a total score that was divided at the median into higher or equivalent versus lower (reference category) exposure groups. We also tested higher centile cut-off values for cleaning product variables. Eco-friendly products comprised one of the queried products, for which we created a separate variable. Based on original response values, categories for eco-friendly products were no use (reference), less than monthly use or at least monthly use. We included questions on the home environment, such as indoor smoking (parents and visitors), number of siblings, household pets and maternal history of asthma and allergy. Maternal body mass index (BMI) was calculated from measured height and pre-pregnancy weight in the perinatal section of the hospital birth record; overweight was defined as BMI ≥ 25.23
Fecal microbiota profiles
Fecal samples were collected at 3–4 months of age. Full details of fecal microbiota profiling can be found in Appendix 1c.
Overweight outcomes
Trained research assistants measured weight and height at 1 and 3 years of age, and generated age- and gender-adjusted BMI z scores from World Health Organization growth charts.24 We categorized weight status at 1 and 3 years of age into overweight or obese (> 97th centile) from generated BMI z scores.
Statistical analysis
We conducted statistical analyses using SPSS software (version 23; IBM SPSS Statistics). The distribution of putative covariates by category of cleaning product was assessed by χ2. The Mann–Whitney U test was employed to compare the median relative abundance, richness and diversity of dominant bacterial taxa. We corrected multiple comparisons by converting crude p values to false discovery rate values (q values).
We determined associations between household cleaning product categories and infant gut microbial composition and diversity with logistic regression analysis, testing bivariate and quintile categories of cleaning product use. We selected potential confounding variables based on the literature25 and on p values (< 0.2) after univariate comparisons. We adjusted models for covariates such as fecal sample age, birth mode, breastfeeding status and antibiotic exposure (infant and maternal intrapartum antibiotic prophylaxis) and presence of the “other” product (for example, models for disinfectant use were adjusted for detergent use).
We also conducted multiple logistic regression modelling to test associations between cleaning product use and overweight or obesity at age 1 and 3 years. When the outcome was BMI z score, we employed multiple linear regression analysis. We adjusted models for the above covariates when relevant. Lastly, using the R package for mediation, we employed causal mediation analysis to test whether cleaning product use (X) affected the overweight outcome (Y) through a microbiota intermediate variable (M).26 Full details of the mediation analysis can be found in Appendix 1c.
Ethics approval
The study was approved by the Human Ethics Boards of the Universities of Alberta, Manitoba and British Columbia; written, informed consent was obtained from all participants.
Results
Data on fecal microbial profiles and prescription records were analyzed in 757 hospital-born infants selected from 3296 infants recruited into the CHILD general birth cohort (Figure 1). Our study sample was representative of the sociodemographics of the larger CHILD cohort (Appendix 1d), apart from small differences in breastfeeding and use of antibiotics in infants, which were adjusted for in models of cleaning product use. High correlations between exclusivity of breastfeeding with maternal education level and study site precluded their addition to models but enabled adjustment for these 2 covariates through the strong proxy measure of breastfeeding status. We excluded maternal pre-pregnancy overweight from the final model for disinfectants and excluded household pet exposure from the final model for eco-friendly products because these covariates did not change the adjusted odds ratio (AOR) estimate > 15%.27,28
Patterns of household cleaning product use
Parental self-report of use of cleaning products was highly correlated with the research assistants’ visual assessment of the presence or absence of those cleaning products in the home (r = 0.32, p = 0.0001). The most common household disinfectant was a multisurface cleaner (22%), whereas handwashing detergents (26%) and spray air fresheners (18%) were the most commonly used in their categories. Close to 80% of households used multisurface cleaners on a weekly to daily basis, and this usage rose to 90% when the cutoff score for high use was the top 30th centile (Appendix 1e). Frequency of household disinfectant usage was significantly correlated with detergent usage (r = 0.450, p = 0.0001), but weakly and inversely with use of eco-friendly products (r = −0.032, p = 0.047). Significant correlations were found between pre - and postnatal use of eco-friendly cleaning products (r = 0.62, p = 0.0001), and pre- and postnatal use of disinfectants (r = 0.60, p = 0.0001).
Disinfectant use was higher at the Edmonton study site and among households with infants delivered by cesarean, who received intrapartum antibiotic prophylaxis, or who were exposed to cigarette smoke, but lower among households with breastfed infants (Table 1). Eco-friendly products were used more often by mothers who had allergies, breastfed their infants, or had higher education, and at the Vancouver study site, and less often in women who were overweight pre-pregnancy or whose infants were admitted to hospital after birth. Associations with use of detergents or other chemicals are shown in Appendix 1f. Exclusive breastfeeding, a covariate related to several cleaning product types, was much more likely among women who were university educated (p < 0.0001) or who lived in Vancouver (p < 0.0001).
Distribution of status of exposure to disinfectant and eco-friendly products at 3–4 months, according to study covariates*
Association of cleaning product use with whole gut microbial community measures
The impact of cleaning products (median or higher v. below) on microbial community composition (beta diversity) was significant for disinfectant use (p = 0.03) but not for use of eco-friendly products (p = 0.1), detergents (p = 0.10) or other cleaning products (p = 0.1). However, gut microbiota richness (Chao1 Index) and diversity (Shannon or Simpson) did not differ between higher and lower frequency of use for any of the cleaning product groups (Appendices 1g–1j).
Disinfectants
The gut microbiota of infants living in homes with higher (≥ median) use of disinfectant products were enriched in Lachnospiraceae (3.320% v. 1.197%, AOR 1.34, 95% confidence interval [CI] 1.02 to 1.90) and its genus Ruminococcus (0.039% v. 0.008%, AOR 1.55, 95% CI 1.10 to 2.17), as well as Coriobacteriaceae (0.047% v. 0.031%, AOR 1.47, 95% CI 1.05 to 2.06) (Figure 2, Table 2, Appendix 1g). They had reduced abundance of fecal Pasteurellaceae (0.015% v. 0.046%, AOR 0.67, 95% CI 0.48 to 0.95) and its genus Haemophilus (0.015% v. 0.046%, AOR 0.69, 95% CI 0.49 to 0.98), as well as genus Clostridium (0.015% v. 0.054%, AOR 0.61, 95% CI 0.43 to 0.86). We did not see these associations with other cleaning products (Appendix 1k).
Composition of key gut microbiota at the family level, by exposure to A) household disinfectant and B) eco-friendly products in all infants (n = 757). The stacked bar charts show mean relative abundance of gut microbiota populations at the family level in infant feces at 3 months of age. A) Left to right, binary category of exposure to disinfectant (≥ median score) and disinfectant exposure in quintiles. B) Left to right, binary category of exposure to eco-friendly products (≥ median score) and questionnaire category of use of eco-friendly products. Asterisks show p values < 0.05 from median relative abundance comparisons from Appendices 1g–1h (median relative abundance with interquartile range comparisons can be found in Appendices 1g–1h).
Crude and adjusted odds ratios for higher abundance (≥ median) of key infant gut microbiota at 3–4 months with frequent household use of disinfectants
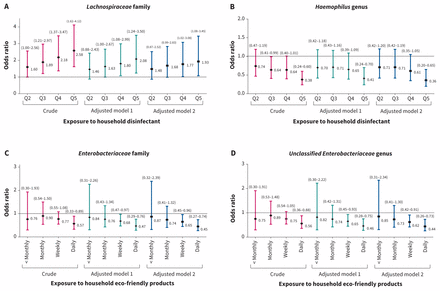
Quintile of household disinfectant use and fecal abundance of Lachnospiraceae were positively associated in a dose-dependent manner (Figure 2), strongest at the highest level of use (AOR 1.93, 95% CI 1.08–3.45) (Figure 3A). An inverse association with genus Haemophilus was strongest at the highest level of household disinfectant use (AOR 0.36, 95% CI 0.20 to 0.65) (Figure 2 and Figure 3B). The abundance of Lachnospiraceae and Pasteurellaceae was negatively correlated (r = −0.194, p = 0.00001, Spearman test). At the genus level, abundance of Ruminococci (of Lachnospiraceae), was negatively correlated with genus Haemophilus (of Pasteurellaceae) (r = −0.157, p = 0.002) or Clostridium (r = −0.122, p = 0.01).
Crude and adjusted likelihood ratios for higher abundance (≥ median) of infant gut microbiota: A) Lachnospiraceae family, B) Haemophilus genus at 3–4 months with frequent household use of disinfectants, C) Enterobacteriaceae family, and D) unclassified Enterobacteriaceae genus at 3–4 months with frequent household use of eco-friendly products. A) and B): Model 1: Adjusted for mode of delivery, breastfeeding status, direct and indirect exposure to antibiotics in first 3 months. Model 2: Adjusted for mode of delivery, breastfeeding status, direct and indirect exposure to antibiotics, exposure to household detergent in first 3 months and fecal sampling age. Q2, Q3, Q4, Q5 = quintiles of household disinfectant exposure (ref: Q1 = lowest quintile). C and D): Model 1: Adjusted for mode of delivery, breastfeeding status, direct and indirect exposure to antibiotics in first 3 months. Model 2: Adjusted for mode of delivery, breastfeeding status, direct and indirect exposure to antibiotics, exposure to household detergent and disinfectant in first 3 months, maternal allergy during pregnancy, maternal overweight and fecal sampling age. Daily, weekly, monthly, < monthly = frequency of use of household eco-friendly products (ref: do not use eco-friendly products).
Eco-friendly products
Infants residing in homes with more frequent use of eco-friendly products had reduced fecal abundance of Enterobacteriaceae (16.364% v. 20.335%, AOR 0.62, 95% CI 0.45 to 0.87) and its genus unclassified Enterobacteriaceae (16.043% v. 20.131%, AOR 0.60, 95% CI 0.43 to 0.83) (Figure 2, Table 3, Appendix 1h). An inverse dose–response was apparent between frequency of use of eco-friendly products and fecal abundance of Enterobacteriaceae, with daily use being associated with the greatest depletion of these microbiota (AOR 0.45, 95% CI 0.27 to 0.74) (Figure 2, Figure 3C). We did not see this association with other cleaning products.
Crude and adjusted odds ratios for higher abundance (≥ median) of key infant gut microbiota at 3–4 months with frequent household use of eco-friendly products
Detergents and other cleaning products
Infants living in homes with higher use of detergents had higher abundance of Erysipelotrichaceae (0.031% v. 0.007%, AOR 1.63, 95% CI 1.16 to 2.29) (Appendices 1i and 1l), but there was no significant dose–response. We did not see this association with other cleaning products (Appendix 1k). We saw no changes in taxon median abundance in infant gut microbiota with frequency in use of other cleaning products, after adjustment for other covariates (Appendices 1j and 1m).
Associations of use of cleaning products and gut microbiota with overweight or obesity
Overweight in children aged 3 years was more prevalent after maternal overweight before pregnancy, cesarean delivery, intrapartum antibiotic prophylaxis, household tobacco exposure and infant antibiotic treatment; it was less prevalent among infants who were exclusively breastfed, whose mothers were more highly educated, or who were at the Vancouver study site (Appendix 1n).
In unadjusted analyses, use of household disinfectant greater than the median was not significantly associated with BMI z score (difference in z score 0.12, 95% CI −0.03 to 0.26) or overweight or obesity at age 3 years (OR 1.66, 95% CI 0.98 to 2.80) (Table 4). However, the top 30th centile of disinfectant use was associated with higher BMI z score (difference in z score 0.17, 95% CI 0.01 to 0.33), although not with overweight or obesity (OR 1.32, 95% CI 0.78 to 2.25) (Table 4). Household use of eco-friendly products greater than the median was associated with a lower BMI z score (difference in z score −0.25, 95% CI −0.40 to −0.11) and reduced odds of overweight or obesity at age 3 years (OR 0.36, 95% CI 0.20 to 0.63). We observed no significant associations between use of household detergents and BMI z score, overweight or obesity.
Crude and adjusted odds ratios for overweight or obesity at 3 years of age with frequent household use of disinfectant and eco-friendly products
In unadjusted analyses, higher fecal levels of Lachnospiraceae at age 3–4 months were significantly associated with increased BMI z score at age 1 (difference in z score 0.29, 95% CI 0.13 to 0.45) and at age 3 (difference in z score 0.28, 95% CI 0.14 to 0.43) (Appendix 1o). In addition, higher fecal levels of Coriobacteriaceae, Erysipelotrichaceae and Ruminococcaceae were associated with increased BMI z score at age 1 and age 3, and higher fecal levels of Enterococcaceae and Clostridiaceae were associated with increased BMI z score at age 3 but not age 1. However, only higher fecal levels of Lachnospiraceae were associated with overweight or obesity at 3 years (OR 1.79, 95% CI 1.06 to 3.04) (Appendices 1o and 1p).
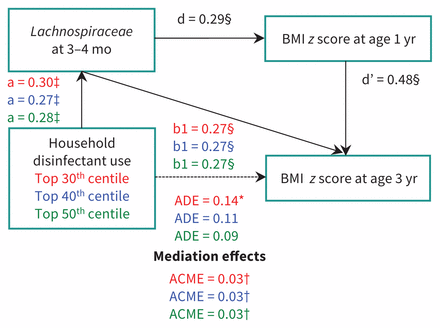
After adjustment for higher fecal abundance of Lachnospiraceae and other covariates, the association between top 30th centile of disinfectant use and BMI z score was no longer significant (difference in BMI z score 0.11, 95% CI −0.02 to 0.25) (Table 4, Appendix 1q). Results were unchanged with the addition of fecal Coriobacterial, Erysipelotrichiaceal and Ruminococcaceal abundance to this model (data not shown). Together, association of disinfectant use with greater Lachnospiraceae abundance, association of Lachnospiraceae abundance with BMI z score or overweight at age 3, and diminishment of the association of disinfectant use with BMI z score after adjustment for Lachnospiraceae abundance suggested a mediation effect by this family of microbiota. Mediation analysis confirmed that Lachnospiraceae abundance had a significant average causal mediation effect on the association of top 30th centile disinfectant use with age 3 BMI z score (p = 0.02) and with overweight status (p = 0.04) (Figure 4, Appendix 1r).
Hypothetical pathway for the association between exposure to household disinfectant and body mass index (BMI) z score at age 3 years. a, b1, ADE, ACME are taken from Appendix 1h; d is taken from Appendix 1g; d′ is taken from Table 4 (a). a = association between exposure to disinfectant and Lachnospiraceae; b1 = association between Lachnospiraceae and BMI z score at age 3 years; d = association between Lachnospiraceae and BMI z score at age 1 year; d′ = association between BMI z score at age 1 year and BMI z score at age 3 years; total effect (a total effect of X [disinfectant/eco-friendly products]) on Y (overweight/obese or BMI z scores 3 years) without mediator (M; Lachnospiraceae/Enterobacteriaceae). ADE (a direct effect of X [disinfectant/eco-friendly products]) on Y (overweight/obese or BMI z scores 3 yr) after taking into account a mediation (indirect) effect of M (Lachnospiraceae/Enterobacteriaceae). ACME (mediation effect) = total effect – ADE.*p < 0.1, †p < 0.05, ‡p < 0.1, §p < 0.001.
After adjustment for fecal abundance of Enterobacteriaceae and other covariates, the association of use of eco-friendly products greater than the median with BMI z score at age 3 was no longer significant (difference in z score −0.12, 95% CI −0.24 to 0.002), but the association with child overweight remained significant (AOR 0.44, 95% CI 0.22 to 0.86) (Table 4). Fecal Enterobacteriaceae did not significantly mediate associations of greater use of eco-friendly products with BMI z score (p = 0.8) or with child overweight (p = 0.2; Appendix 1r). However, among vaginally born infants with a lower abundance of Enterobacteriaceae microbes at age 3–4 months, greater use of eco-friendly products was associated with decreased odds of overweight or obesity at age 3 (AOR 0.24, 95% CI 0.06–0.86); no associations were found in infants delivered by cesarean (Appendix 1s).
Interpretation
In a subsample of 757 Canadian infants, derived from a population-based birth cohort, household cleaning product use affected gut microbiota at 3–4 months of age, independently of infants’ exposure to antibiotics, birth mode, breastfeeding and other microbe-altering covariates. Associations with altered microbiota were most compelling for frequent use of household disinfectants, which showed reduced abundance of genus Haemophilus and of genus Clostridium. These changes are compatible with the bacterial-killing actions of disinfectants containing bleach and hydrogen peroxide.29,30 At the same time, Lachnospiraceae were 1.3 times more likely to be overrepresented in infant gut microbiota after frequent cleaning with disinfectants. This enrichment with Lachnospiraceae at 3–4 months of infant age strongly predicted a higher BMI z score at age 1 which, in turn, was a strong determinant of BMI z score at age 3. Moreover, we found evidence of statistical mediation by fecal Lachnospiraceae of the association between weekly to daily cleaning with disinfectant during infancy and an increase in BMI z score at age 3.
Infant fecal abundance of Lachnospiraceae rose with frequency of disinfectant cleaning in a dose-dependent manner, with 2-fold higher odds of higher microbial abundance in the highest usage category. Commonly found in infants with detected Lachnospiraceae,31 genus Ruminococcus became 1.6 times more likely to have higher abundance with frequent use of disinfectants in our study infants, in conjunction with lowered abundance of genus Haemophilus and Clostridium. This same compositional profile is typical of eczema in children.32 Elevated fecal abundance of Lachnospiraceae (specifically Blautia) concurrent with lowered Haemophilus is also a signature of diabetes, as shown in a study on 11-year-old children.33 Blooms in Lachnospiraceae have been observed with subtherapeutic doses of antibiotic treatment in a murine model of obesity34 and in newborn piglets after environmental aerosolization with a disinfectant.9 Greater prominence of the Lachnospiraceae or individual species in gut microbiota has been associated with higher visceral white adipose tissue mass and insulin resistance in mice,35 and with higher body fat and insulin resistance in human adults.36
Genus Haemophilus were further depleted when disinfectants were employed on a daily basis, consistent with their exquisite sensitivity to high concentrations of hydrogen peroxide37 and frequent use of household wipe products.38,39 Unlike in the infant nasopharynx,40 Haemophilus species are low-abundant microbiota in the gut;41,42 they decline, but genus Clostridium becomes more prominent as full-term and even hospitalized preterm infants get older. Less well studied than Clostridium difficile colonization, 43,44 other species of Clostridium, such as clusters XIVa and IV, are important to gut motility, water absorption and immune tolerance.45 Our observations of both genera are remarkably consistent with the study by Schmidt and colleagues, in which the postbirth transfer of piglets to a pathogen-free environment, aerosolized with disinfectant, caused a reduction in intestinal tissue levels of Pasteurellaceae and Clostridiaceae, and a rise in Lachnospiraceae.9 Increases to Enterobacteriaceae were also seen in these piglets.
On the other hand, infants remained normal weight when eco-friendly products were used daily and Enterobacteriaceae were less abundant in their gut. Eco-friendly products have efficacy against Escherichia coli;12 when these microbes are fewer in number, adiposity is less likely in toddlers.46 However, we found no statistical evidence that Enterobacteriaceae mediated the strong association between postnatal use of eco-friendly products, and BMI z score or child overweight. This led us to speculate about lower rates of transmission for Enterobacteriaceae during vaginal birth when mothers used eco-friendly products or led healthy lifestyles.47 In support of this thesis, we identified an inverse association between high use of eco-friendly products and child overweight only among infants with low levels of Enterobacteriaceae and who had been born vaginally.
This large, general-population birth cohort study tested associations between home cleaning products and gut microbial composition in early life, and later overweight, which were adjusted for microbe-altering covariates. We employed high-throughput genetic sequencing to profile whole fecal microbial communities. These aspects enhanced the external and internal validity of the results. Associations between cleaning product use and infant gut microbial changes were dose dependent, and showed consistency with porcine and murine model experiments, meeting 2 additional Bradford Hill criteria for causation. Finally, tests for mediation contributed evidence on the relevance of microbiota changes to the development of child overweight.
Limitations
The status of infant exposure to cleaning agents was assumed from parent report. Recall bias is thus a possibility. Nonetheless, most questions on use of household cleaning products were adapted from the Seattle–King County Healthy Homes study, which has shown the effectiveness of household interventions in reducing these exposures in children.48 Our study did not differentiate cleaning products by brand name or the presence of specific ingredients. The latter is challenging as some ingredients are not listed on product labels, especially of eco-friendly products. Finally, infant gut microbiota were profiled at a single time point, although the fecal sample was obtained during a critical early phase of infant development.
Conclusion
Antibacterial cleaning products have the capacity to change the environmental microbiome and alter risk for child overweight. Our study provides novel information regarding the impact of these products on infant gut microbial composition and outcomes of overweight in the same population. We found Lachnospiraceae to be enriched in infant gut microbiota with frequent postnatal use of domestic disinfectants but not eco-friendly products; genus Clostridium and Haemophilus were reduced concurrently. Evidence of statistical mediation with Lachnospiraceae abundance showed a role for this disinfectant-related change to gut microbiota in causing overweight. We did not observe mediation for infant fecal Enterobacteriaceae, suggesting an alternate pathway for the association between postnatal eco-friendly product use and reduced child overweight. Further study is required on the mechanisms through which household cleaning products alter gut microbial composition and the subsequent role this might have on metabolic disease.
Acknowledgements
The authors are grateful to all the families who took part in this study, and the whole CHILD team, which includes interviewers, data and laboratory technicians, clerical workers, research scientists, research assistants, volunteers, managers, receptionists and nurses.
Footnotes
CHILD investigators: Padmaja Subbarao (Director), The Hospital for Sick Children; Stuart Turvey (Co-Director), University of British Columbia; Malcolm Sears (Founding Director), McMaster University; Sonia Anand, McMaster University; Meghan Azad, University of Manitoba; Allan Becker, University of Manitoba; Dean Befus, University of Alberta; Michael Brauer, University of British Columbia; Jeff Brook, University of Toronto; Edith Chen, Northwestern University, Chicago; Michael Cyr, McMaster University; Denise Daley, University of British Columbia; Sharon Dell, The Hospital for Sick Children; Judah Denburg, McMaster University; Qingling Duan, Queen’s University; Thomas Eiwegger, The Hospital for Sick Children; Hartmut Grasemann, The Hospital for Sick Children; Kent HayGlass, University of Manitoba; Richard Hegele, The Hospital for Sick Children; Linn Holness, University of Toronto; Perry Hystad, Oregon State University; Michael Kobor, University of British Columbia; Tobias Kollmann, University of British Columbia; Anita Kozyrskyj, University of Alberta; Catherine Laprise, Université du Québec à Chicoutimi; Wendy Lou, University of Toronto; Joseph Macri, McMaster University; Piush Mandhane, University of Alberta; Gregory Miller, Northwestern University, Chicago; Theo Moraes, The Hospital for Sick Children; Peter Paré, University of British Columbia; Clare Ramsey, University of Manitoba; Felix Ratjen, The Hospital for Sick Children; Andrew Sandford, University of British Columbia; James Scott, University of Toronto; Jeremy Scott, University of Toronto; Frances Silverman, University of Toronto; Elinor Simons, University of Manitoba; Tim Takaro, Simon Fraser University; Scott Tebbutt, University of British Columbia; Teresa To, The Hospital for Sick Children.
CMAJ Podcasts: author interview at https://soundcloud.com/cmajpodcasts/170809-res
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Mon Tun performed statistical analyses, prepared figures and tables, and wrote the first draft of the manuscript. Anita Kozyrskyj conceived the study, obtained funding, planned the data analysis and wrote the final version of the manuscript. Theodore Konya conducted DNA extraction and sample preparation for sequencing. Hein Tun generated gut microbiota operational taxonomic unit profiles using QIIME software. Wendy Lou and Justin Mahoney provided statistical advice. David Guttman, Malcolm Sears, Allan Becker, Piush Mandhane, Padmaja Subbarao, Stuart Turvey, Jeffrey Brook, Tim Takarao and James Scott obtained funding, advised on the study design or coordinated data collection. All the authors provided critical comments on the manuscript content and approved the final version of the manuscript. Anita Kozyrskyj and James Scott will serve as guarantors for the manuscript’s contents.
Funding: The Canadian Institutes of Health Research (CIHR) and the Allergy, Genes and Environment (AllerGen) Network of Centres of Excellence provided core support for the CHILD study. The study was specifically funded by the CIHR Microbiome Initiative team grant no. 227312.
Data sharing: Data requests from interested researchers can be made to the CHILD study through the director, Padmaja Subbarao (padmaja.subbarao{at}sickkids.ca), or the research manager, Diana Lefebvre (efeb{at}mcmaster.ca).
- Accepted June 25, 2018.














Podcast