Abstract
BACKGROUND: Oral morphine for postoperative pain after minor pediatric surgery, while increasingly popular, is not supported by evidence. We evaluated whether oral morphine was superior to ibuprofen for at-home management of children’s postoperative pain.
METHODS: We conducted a randomized superiority trial comparing oral morphine (0.5 mg/kg) with ibuprofen (10 mg/kg) in children 5 to 17 years of age who had undergone minor outpatient orthopedic surgery (June 2013 to September 2016). Participants took up to 8 doses of the intervention drug every 6 hours as needed for pain at home. The primary outcome was pain, according to the Faces Pain Scale – Revised, for the first dose. Secondary outcomes included additional analgesic requirements, adverse effects, unplanned health care visits and pain scores for doses 2 to 8.
RESULTS: We analyzed data for 77 participants in each of the morphine and ibuprofen groups. Both interventions decreased pain scores with no difference in efficacy. The median difference in pain score before and after the first dose of medication was 1 (interquartile range 0–1) for both morphine and ibuprofen (p = 0.2). For doses 2 to 8, the median differences in pain score before and after the dose were not significantly different between groups. Significantly more participants taking morphine reported adverse effects (45/65 [69%] v. 26/67 [39%], p < 0.001), most commonly drowsiness (31/65 [48%] v. 15/67 [22%] in the morphine and ibuprofen groups, respectively; p = 0.003).
INTERPRETATION: Morphine was not superior to ibuprofen, and both drugs decreased pain with no apparent difference in efficacy. Morphine was associated with significantly more adverse effects, which suggests that ibuprofen is a better first-line option after minor surgery.
Trial registration: ClinicalTrials.gov, no. NCT01686802.
Moderate to severe pain is the most common postoperative pediatric complaint,1 particularly within 24 hours2 and even after minor surgery.3–7 Inadequate analgesia is prevalent after outpatient pediatric surgery3,6–10 and is the most common reason for unplanned hospital admissions.11 In children, suboptimal analgesia can result in sleep disturbance, behavioural changes and vomiting.12 Untreated pain leads to slower wound healing,13 needle phobia,14 hyperesthesia15 and fear of medical procedures.16
Undertreatment is an important cause of inadequate postoperative analgesia.17–20 Both ibuprofen21,22 and oral morphine22,23 have shown benefit in children with musculoskeletal injuries. Following deaths of children who received codeine postoperatively,24,25 the United States Food and Drug Administration26 and Health Canada27 issued advisories. Orthopedic procedures are associated with the highest incidence of post-discharge pain.28,29 Although not discipline-specific, more than 80% of pediatric surgeries are performed on an outpatient basis,30 which renders pain management largely the responsibility of caregivers at home. Oral morphine and other opioids are being prescribed more often.31–33 However, evidence supporting morphine for at-home postoperative pain management in children is lacking.
Given the lack of consensus standards for analgesia after pediatric outpatient surgery, suboptimal provision of analgesia2,9 and growing fears about opioids, there is an urgent need for evidence to guide outpatient analgesic choices for children at discharge. We sought to evaluate whether oral morphine was superior to ibuprofen for relieving children’s pain at home, after minor outpatient orthopedic surgery.
Methods
Design and setting
For this parallel-group, randomized, blinded superiority trial, we recruited participants from June 2013 to September 2016 at the Children’s Hospital, London Health Sciences Centre, London, Ontario, where about 150 pediatric outpatient orthopedic surgeries are performed annually. A data and safety monitoring board convened at 25%, 50% and 75% recruitment to monitor adverse effects.
Participants
We included all children aged 5 to 17 years who presented to the pediatric orthopedic clinic and were scheduled for minor outpatient surgery. We excluded children with known hypersensitivity to ibuprofen or morphine, long-term use of nonsteroidal anti-inflammatory drugs (NSAIDs) or opioids, renal insufficiency, bleeding disorder, cognitive impairment, obstructive sleep apnea, regional anesthesia or pregnancy.
Patients were screened consecutively for eligibility by a research assistant, who obtained informed consent and assent from all participants or their legal guardians, and who performed all study-related correspondence with participants.
Interventions
The hospital pharmacy performed randomization, using a computer-based random number generator (www.randomization.com). Eligible participants were randomly assigned in a 1:1 allocation ratio using a block size of 4 or 6 to receive either standard-release oral morphine (0.5 mg/kg, maximum 20 mg) or oral ibuprofen (10 mg/kg, maximum 600 mg) every 6 hours as needed for pain, for a total of 48 hours after discharge (maximum 8 doses). Allocation was concealed through use of sequentially numbered, opaque sealed envelopes. Study medications and placebos were kept in identical opaque sealed bottles and were dispensed in identical white plastic vials. Because of differences in taste and consistency, we employed a double-dummy approach34 whereby each participant was given 8 prepackaged doses consisting of 2 vials (morphine and placebo ibuprofen or placebo morphine and ibuprofen). The placebos were identical in taste, colour and consistency to their active drug counterparts, so that participants were unaware of which intervention they were receiving. A protocol for unmasking was available on an emergency basis. Participants were asked to take a second dose if they vomited within 30 minutes of the first. For pain persisting longer than 60 minutes after the intervention, participants were instructed to take acetaminophen 15 mg/kg (maximum of 975 mg).
Participants recorded their pain using the Faces Pain Scale – Revised35 at discharge and at home (immediately before and 30 min after each dose). Thirty minutes is the time to a clinically significant reduction in pain,23,36 to peak plasma concentration for oral morphine37 and to onset of analgesia for ibuprofen.38 Consistent with the described use of the Faces Pain Scale – Revised,39 we instructed all children to circle the face on the horizontal axis that corresponded to their pain level. We asked caregivers to supervise their children to ensure that only 1 face was circled at each time point. Caregivers and participants also recorded the number of acetaminophen doses taken for pain and any adverse effects that occurred within 96 hours of the first dose (selected from a list of known adverse effects).
Participants returned data collection forms by mail, using a stamped, self-addressed envelope, and returned unused medication to the study coordinator at the follow-up orthopedic clinic visit. We corroborated the reported number of doses taken with the medication that remained on the follow-up visit. We contacted participants whose forms contained unclear information. Discrepancies were resolved by discussion with the caregivers. A research assistant entered data from the forms into a study-specific Excel spreadsheet. Participants received a phone call at 24, 48, 72 and 96 hours after discharge to inquire about severe adverse events and unscheduled visits to a health care provider. Participants, caregivers and all members of the research team were blinded to the interventions.
Anesthesia and analgesia before discharge were provided as per the standard of care at our institution. Intraoperatively, participants received a weight-based dose of fentanyl and propofol with or without lidocaine for induction and sevoflurane for maintenance of anesthesia. At the end of the case, each participant received a subcutaneous injection of 2–5 mL of 1% lidocaine around the incision site. In the postanesthetic care unit, each participant received a weight-based dose of acetaminophen or, if the patient was in more severe pain, intravenous (IV) morphine.
Outcome measures
The primary outcome was the pre–post difference in self-reported pain for the first dose, based on the pain scale.35 The Faces Pain Scale – Revised has been validated for postoperative use40 in children from 4 to 12 years of age39 and is believed to be clinically useful for older children.41 Although not validated for home use, the scale has been used at home for fracture pain22 and postoperative pain,2 is preferred by children,40 does not require training42 and was easily interpretable across our sample’s age range.41 The 6-item Faces Pain Scale – Revised is scored from 0 (no pain) to 10 (maximum pain), with severity defined as none (0 or 2), mild (4), moderate (6) or severe (8 or 10).43 Secondary outcomes included the pre–post difference in pain for the second to eighth doses (also based on the pain scale), the number of participants requiring breakthrough acetaminophen for pain, unscheduled visits to a health care provider for pain within 96 hours of the first dose and adverse effects.
Statistical analysis
We analyzed between-group differences in pain scores using an intention-to-treat analysis, based on the assumption that for participants who received no study intervention, the pre–post difference in pain scores would be zero. A difference in pain intensity of 1 face on the Faces Pain Scale – Revised has been shown to be a minimal clinically important difference.43,44 We analyzed the between-group differences in pain scores using the Wilcoxon 2-sample test. We used a mixed linear regression model, with an unstructured covariance matrix, to assess between-group differences in pain scores across all doses over time. We used the Pearson χ2 test or Student t test to evaluate differences in acetaminophen use and adverse effects. The study was powered to detect a between-group difference in the primary outcome. Assuming a standard deviation of 2 faces,39,41 63 children per group were required to detect a between-group difference of 1 face at the 5%, 2-sided level of significance with 80% power.45 We used SPSS software, version 23 (IBM). We considered p values less than 0.05 to be statistically significant.
Ethics approval
This trial was approved by the Health Sciences Research Ethics Board of Western University.
Results
Participants
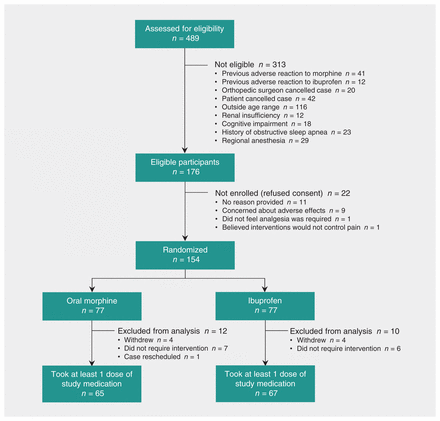
We report results of an intention-to-treat analysis of 154 participants, of whom 71 (46%) were female (Figure 1, Table 1). The mean age was 12.4 (standard deviation [SD] 3.5) years. The overall mean pain score at discharge was 2.3 (SD 1.3) (Table 1). No participants were lost to follow-up. Eight participants withdrew after randomization because they believed that ibuprofen would not adequately manage pain. Thirteen participants did not take any study medication, and all of these reported that the pain had not been sufficiently severe. All study vials were returned, and all opened vials were empty. For all but 1 participant, the number of used vials corresponded to the information on the data collection form. For the 1 exception, the participant reported a single pair of pain scores independent of analgesic administration. There were no obvious between-group differences in surgical procedures performed. No participants received intra-articular analgesia, regional analgesia, clonidine or long-acting analgesics. Three participants (2 in the morphine group and 1 in the ibuprofen group) received a single dose of IV morphine 0.05 mg/kg in the postanesthetic care unit. The demographic characteristics of participants who underwent randomization and did not take any study medication are presented in Table 2.
Flow of participants through the trial.
Demographic characteristics of participants
Characteristics of participants according to whether data were included in analysis*
Primary outcome
The median time to the first dose was 3.3 (interquartile range [IQR] 0.5–6) hours for the morphine group and 3.5 (IQR 1–6) for the ibuprofen group. There were no significant differences between groups in the change in pain scores for the first dose (p = 0.2) (Table 3). There were no significant between-group differences in pain scores over time (p = 0.4).
Pain scores before and after each dose of pain medication, according to the Faces Pain Scale — Revised, and difference in score
Secondary outcomes
Sixty-five (84%) and 67 (87%) of participants took at least 1 dose of oral morphine or ibuprofen, respectively. There were no significant differences in the change in pain scores for doses 2 to 8. However, both morphine and ibuprofen produced a decrease in pain scores with each dose (Table 3). There was no significant difference in the number of participants who required acetaminophen for breakthrough pain (p = 0.2). Among participants who took acetaminophen, there was no significant difference in the number of acetaminophen doses taken per participant (p = 0.09) (Table 4). Significantly more participants in the morphine group than in the ibuprofen group experienced adverse effects (45/65 [69%] v. 26/67 [39%], p < 0.001; Table 4). There were no serious adverse events, deaths, unscheduled visits to a health care provider for pain or adverse events, or unmasking of the intervention.
Adverse effects and requirement for breakthrough acetaminophen for participants who took at least 1 dose of the study drug
Interpretation
In this trial of at-home pain management in children who underwent minor orthopedic surgery, both oral morphine and ibuprofen reduced pain with no apparent difference in analgesic efficacy. Most of the children experienced pain severe enough to require analgesia. Oral morphine was associated with significantly more adverse effects, which suggests that ibuprofen is a safer first-line analgesic.
Among children who have undergone ambulatory surgery, oral morphine has not been studied for at-home therapy, nor has it been compared with ibuprofen, a frequently used analgesic. Our results are consistent with those from a study of children with non-operative fractures in which oral morphine produced analgesia comparable to that with ibuprofen but had significantly more adverse effects.22 In children remaining in hospital after surgery, several studies have found that parenteral46 and oral morphine47,48 did not produce better analgesia than active comparators. Our findings contribute to this evidence, because we have shown that after discharge from ambulatory surgery, oral morphine has no analgesic advantage over ibuprofen for first-line treatment of pain.
In contrast to our findings that ibuprofen was associated with clinically significant pain reduction,39 a systematic review of NSAIDs for children in the postanesthetic care unit suggested otherwise.49 Unlike patients receiving care in hospital, our sample included children who underwent minor surgeries that may not have been associated with severe pain43 and who may have experienced less distress in their home environment. Among pediatric inpatients, anxiety has been found to be highly correlated with postoperative pain.50
In our study, pre-intervention pain scores for the first 6 doses correlated with mild to moderate pain,43 but were associated with a perceived need for analgesia.41,51 For the first 6 doses, both agents produced a modest but clinically important reduction in pain.39 However, most post-intervention pain scores remained above the analgesic threshold recommended by the World Health Organization.51 Consistent with our work, neither ibuprofen nor oral morphine (nor a combination) has shown complete efficacy in children with musculoskeletal injury.52 We also found that more than 80% of children required analgesia in the first 24 hours. This result suggests that adequate pain management should be an important goal of care, even after minor outpatient surgery, and that more effective pharmacologic and nonpharmacologic strategies should be explored.
The most common adverse effects associated with morphine were drowsiness (48%) and nausea (46%), in keeping with previous studies.22,33,46 Among pediatric patients with obstructive sleep apnea who underwent tonsillectomy, Kelly and colleagues48 described a significantly higher frequency of oxygen desaturation with oral morphine. We chose not to measure this outcome because our sample included children with orthopedic pathology and excluded patients with obstructive sleep apnea. Concerns about adverse effects are prevalent (73%) among caregivers of children who undergo outpatient surgery,3 as are concerns regarding the addictive potential of opioids.3,33 These concerns may be fueled by recent pediatric evidence of long-term risks53 and increased hospital admissions related to opioids.54 These reports, together with our findings, suggest that ibuprofen may be more acceptable to caregivers who are managing children’s pain at home.
Finally, access to analgesia is a barrier to effective postoperative pain management.20 Opioids, including morphine, typically require a prescription and a dispensing source. In contrast, ibuprofen is available without a prescription and is relatively inexpensive; furthermore, accidental ingestion and intentional overdose have less severe clinical consequences.
Limitations
The primary limitation of our study was the requirement for a fixed dosing interval (6 h) to preserve blinding. Differences in the duration of action between morphine (2–4 h)37 and ibuprofen (4–8 h)55 could have led to greater analgesic requirements in the morphine group. We found the opposite, however, in that more participants in the ibuprofen group reported using acetaminophen for breakthrough pain. More importantly, median pre-intervention pain scores were virtually identical in the 2 groups. Second, we included participants who underwent a variety of surgical procedures, which may have contributed to baseline heterogeneity. Our study was not powered for secondary outcomes, but we do not believe this imparted any substantial bias, because there were no large differences in discharge or pre-intervention pain scores or in the number of medication doses. Third, the Faces Pain Scale – Revised is a self-reporting tool not validated for home use. However, we believed it to be the single best approach to determining pain levels at home, and it is currently the recommended instrument for the age range of patients included in this study.56–59 In contrast to self-reported measures, observational assessments by health care providers, though arguably more objective, have been found to underestimate children’s postoperative pain.40 Finally, our results may not be applicable to children who have undergone surgical procedures associated with greater than moderate levels of pain.
Conclusion
Both oral morphine and ibuprofen were associated with clinically significant pain reduction in children who underwent minor outpatient orthopedic surgery. Morphine did not provide superior analgesia, but was associated with significantly more adverse effects, making ibuprofen a better analgesic option. Importantly, pain was not completely managed by either intervention. Future work should explore whether combinations of non-opioid analgesics, more potent opioids with fewer adverse effects or nonpharmacologic therapies offer greater benefit, particularly for more severe pain.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Naveen Poonai was the primary investigator and was responsible for designing the study, interpreting and analyzing the data and writing the manuscript. Natasha Datoo, Megan Cashin and Debra Bartley were responsible for designing the study, overseeing data collection and writing the Methods, Results and Interpretation sections of the manuscript. Samina Ali, Amy Drendel and Michael Rieder made extensive contributions to the design of the study, interpretation of the data and revision of the manuscript. Rongbo Zhu was responsible for tracking participants and data entry and contributed to writing the Methods and Results sections of the manuscript. Natasha Lepore and Michael Greff were responsible for participant recruitment, data collection and interpretation, and contributions to writing the Introduction and Methods sections of the manuscript. All of the authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This study was funded by a Schulich Research Opportunities Grant from Western University, London, Ont.
Data sharing: All portions of the data are available for sharing, upon contact with the corresponding author.
- Accepted June 7, 2017.