Abstract
Background: A school-based program with quadrivalent human papillomavirus (HPV) vaccination was implemented in Alberta in 2008. We assessed the impact of this program on Pap test cytology results using databases of province-wide vaccination and cervical cancer screening.
Methods: We conducted a nested case–control study involving a cohort of women in Alberta born between 1994 and 1997 who had at least 1 Pap test between 2012 and 2015. Women with negative cytology results were controls. Women with low-grade (atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion) and high-grade (atypical squamous cells, cannot rule out a high-grade lesion; or high-grade squamous intraepithelial lesion) cervical abnormalities were cases. Exposure status was assigned according to records of HPV vaccination. Odds ratios (ORs) for abnormal cytology results by vaccination status were adjusted for neighbourhood income, laboratory service, rural versus urban residency, and age.
Results: The total study population was 10 204. Adjusting for age, vaccinated women had a higher screening rate than unvaccinated women (13.0% v. 11.4%, p < 0.001). Among women who received full vaccination (≥ 3 doses), the adjusted OR for cervical abnormalities was 0.72 (95% confidence interval [CI] 0.63–0.82). For high-grade lesions, the adjusted OR was 0.50 (95% CI 0.30–0.85). With 2-dose HPV vaccination, the adjusted OR for cervical abnormalities was 1.08 (95% CI 0.84–1.38).
Interpretation: Quadrivalent HPV vaccination significantly reduced high-grade cervical abnormalities but required 3 doses. Vaccination against HPV was associated with screening uptake. Population-based vaccination and screening programs should work together to optimize cervical cancer prevention.
Human papillomavirus (HPV) infection causes cervical dysplasia that can progress into invasive cervical cancer.1 Screening programs for cervical cancer use the Papanicolaou (Pap) test to detect preinvasive cervical abnormalities.1,2 Recently, HPV vaccination programs have been added to efforts to prevent cervical cancer.3,4 Vaccination against HPV should reduce the prevalence of cervical dysplasia; thus, screening outcomes provide early evidence of the effectiveness of HPV vaccination against cancer.5–8 Data are lacking on the association between Pap test results and HPV vaccination in the North American context.9–11 The province of Alberta has a population-based program for cervical cancer screening and HPV vaccination, and is well positioned to address this knowledge gap.
In 2008, Alberta implemented a school-based HPV vaccination program for female students in grade 5 (age 10–11 yr) and, in 2009, added a 3-year catch-up program for female students in grade 9 (age 14–15).12 The HPV vaccination program expanded to include male students in 2014.13 The current program provides 3 doses (months 0, 2 and 6) of quadrivalent vaccine that includes protection against 2 oncogenic HPV types (16 and 18), which together account for 70% of cases of cervical cancer.14,15 The Alberta Cervical Cancer Screening Program (ACCSP) recommends routine screening for cervical cancer with Pap tests beginning at age 21; however, some women elect to begin screening at a younger age.16
The objective of this study was to assess the impact of the Alberta school-based HPV vaccination program on Pap test cytology results using databases of province-wide vaccination and cervical cancer screening. The study population is the first cohort of women in Alberta who were age-eligible for the school-based HPV vaccination program and participated in any cervical cancer screening.
Methods
Alberta has a universal public health care system, available to all residents. Residents of the province are required to register with the Alberta Health Care Insurance Plan, with more than 99% of the population registered.17 A personal health number for each registered person acts as a unique lifetime identifier. This number can be used to support deterministic linkage across administrative health databases.
Study design
Given that the school-based HPV vaccination cohort was screened for cervical cancer opportunistically (i.e., at a younger age than recommended by the ACCSP), we conducted a nested case–control analysis.
Data sources
We collected cytology results from the ACCSP database and collected vaccination records from Alberta’s provincial immunization repository (Immunization and Adverse Reaction to Immunization [Imm/ARI]) and the Pharmaceutical Information Network (PIN).
The ACCSP database receives all of the laboratory test data for women who are residents of Alberta and aged 18 to 69 years. The cervical cancer screening application has linkages to multiple provincial repositories including Pap test laboratory results and cancer registry data.2 Demographic information is obtained from Alberta Health’s Person Directory.18 The cervical cancer screening application undergoes continuous reviews for quality assurance, and its data elements are validated and verified against the multiple provincial information linkages.
The Imm/ARI repository captures all vaccinations administered through public health programs, including those administered through the school-based program. In 2013 and 2014, 74.1% of the public vaccination program’s target population completed 3 doses of HPV vaccination by grade 9.19 The Imm/ARI repository has strict guidelines that promote high-quality data submissions and is believed to capture all publicly funded vaccinations in the province.20
The Pharmaceutical Information Network is used for privately dispensed vaccines to capture women who are age-eligible for the school-based program but vaccinated outside of the program. Less than 5% of the total HPV vaccinations were provided from PIN. We assumed that the vaccination event is an appropriate proxy for the dose administered. More than 95% of pharmacists submit records to PIN,21 and every dispensation record in PIN follows data-quality protocols to ensure the accuracy of information entered at the pharmacy level.
Study population
Our study population included women born between 1994 and 1997, who had at least 1 Pap test between 2012 and 2015, and who had permanent residency in Alberta, as per the ACCSP eligibility criteria. Women identified as First Nations or Inuit, based on the Provincial Registry, were excluded because they were unlikely to have been completely captured within the data sets used in the current analyses. First Nations and Inuit people are indigenous populations in Canada that receive health care services from federally funded health centres for which the province of Alberta has limited information. We also excluded women who moved to Alberta after 2008 and who had no vaccination record. If these groups of women were vaccinated outside of the province, they could have been misclassified as unvaccinated. Those who had only unsatisfactory Pap test results were excluded because their case–control status could not be determined.
Outcome and exposure measures
To assign case–control status, we used the most severe cytology results (reported based on the Bethesda System22) captured in the ACCSP database between Jan. 1, 2012, and Aug. 14, 2015. Women with negative cytology results were the controls, and women with low-grade (atypical squamous cells of undetermined significance [ASC-US] or low-grade squamous intraepithelial lesion [LSIL]) and high-grade (atypical squamous cells, cannot rule out a high-grade lesion [ASC-H]; or high-grade squamous intraepithelial lesion [HSIL]) cervical abnormalities were cases.22 We considered a woman “vaccinated” if there was a record of at least 1 dose of HPV vaccination before the Pap test used for the case–control assignment, “fully vaccinated” if she had 3 or more recorded doses of the HPV vaccination and “partially vaccinated” if fewer than 3 doses were recorded. “Unvaccinated” women were those with no record of vaccination before the Pap test. The first HPV vaccination in the study population took place in 2006, when the vaccine was approved in Canada.12
Covariates
Our covariates included neighbourhood income, urban versus rural residency, the laboratory service that processed the cytologic specimens and age in years (as of July 31, 2015), similar to other studies.6,8 Statistics Canada provided neighbourhood income quintiles based on dissemination area, which was further linked to postal code. Rural residency designation was created by Alberta Health, based on population densities and travel times to a variety of health services. In Alberta, 2 laboratory services in Calgary and Edmonton process cytologic specimens. Collected samples are processed using different technologies (ThinPrep in Calgary; SurePath in Edmonton). Both are considered valid technologies, but SurePath has a slightly higher positive predictive value (PPV).23,24 Given the low prevalence of cervical abnormality in the study population, the results were adjusted for laboratory. There are other known risk factors for HPV infection and cervical cancer, such as smoking.16 However, we could not obtain such data for the study population. Covariates with missing values were excluded from the analysis.
Statistical analysis
We compared the sociodemographic characteristics of cases and controls using a χ2 test. We used logistic regression analysis to regress case–control status on vaccination status to estimate the exposure odds ratio (OR) and 95% confidence interval (CI), and then adjusted for covariates. We calculated vaccine effectiveness as a percentage [(1 – adjusted OR) × 100%]. All statistical tests were 2-sided with a 0.05 level of significance. Data linkage and analyses were conducted using SAS version 9.3.
Results
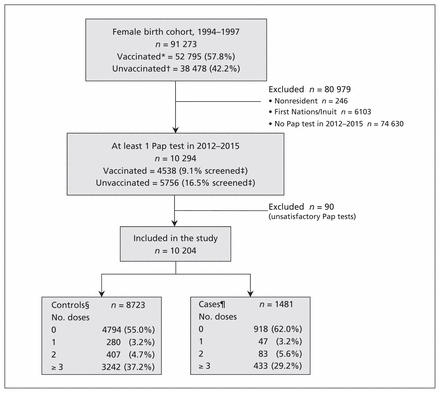
Figure 1 illustrates the selection of our study population. In Alberta, 91 273 women born between 1994 and 1997 were age-eligible for the school vaccination program that started in 2008. From this cohort, 10 204 women were eligible for inclusion in our study: 1481 were cases (14.5%) and 8723 were controls (85.5%). Of the study population, 5712 (56.0%) were unvaccinated and 4492 (44.0%) had at least 1 dose of HPV vaccine before cervical cancer screening, between 2006 and 2015. Adjusting for age, vaccinated women had a higher screening rate than unvaccinated women (13.0% v. 11.4%, p < 0.001). Among the vaccinated women, the median separation between their last vaccination and Pap test was 1374 (interquartile range [IQR] 1119–1603) days. Table 1 details the characteristics by birth cohort.
Flow diagram for population selection. *At least 1 dose of human papillomavirus (HPV) vaccine before first Pap test. †No recorded dose of HPV vaccine before first Pap test. ‡Unadjusted screening rate of vaccinated/unvaccinated cohort. §Negative cytology result. ¶Includes the following cytology results: atypical squamous cells of undetermined significance; low-grade squamous intraepithelial lesion; atypical squamous cells, cannot rule out a high-grade lesion; and high-grade squamous intraepithelial lesion.
Vaccination status, screening rates, and cytology results of the 1994–1997 birth cohort, by year of birth
Among cases, 1384 (93.5%) had low-grade cervical abnormalities and 97 (6.5%) had high-grade cervical abnormalities. Cases were older (p = 0.046) and more likely to have had their Pap tests processed at the laboratory in Calgary (p < 0.001). Cases and controls were similar in regard to neighbourhood income and rural versus urban residency. Table 2 shows the sociodemographic characteristics of cases and controls. Less than 1% of covariates had missing values. Among the vaccinated women, 83.8% completed 3 or more doses of the vaccine. Vaccination rates were higher in younger women: 76.6% of the 18-year-old group had at least 1 dose of HPV vaccine compared with 20.4% of the 21-year-old group (p < 0.001). Table 3 details characteristics of the vaccinated and unvaccinated subpopulations.
Demographic characteristics, laboratory site and vaccination dose of cases and controls
Demographic characteristics, laboratory site and cytology outcome, by vaccination dose
Table 4 summarizes the main study outcomes. Among women who received full HPV vaccination (≥ 3 doses) (compared with no vaccination), the adjusted OR for abnormal cytology results was 0.72 (95% CI 0.63–0.82). Vaccine effectiveness of full HPV vaccination for abnormal cytology results was 28% (95% CI 18%–37%). Among the fully vaccinated women (n = 3675), 11.8% had abnormal cytology results, whereas among unvaccinated women (n = 5712), 16.1% had abnormal cytologic results.
Adjusted odds of cervical abnormalities (worst cytology result),* by vaccination status
Among partially vaccinated women, (i.e., 2-dose vaccination compared with 0 doses), the adjusted OR for abnormal cytology results was 1.08 (95% CI 0.84–1.38), indicating that the 2-dose vaccination was not effective. The adjusted OR for high-grade cervical abnormalities was 0.16 (95% CI 0.02–1.17) among partially vaccinated women. Median separation between first and second doses was 91 (IQR 56–147) days.
We conducted a sensitivity analysis by separating the high-grade and low-grade cases, and redefining controls as negative or low-grade cytologic results to isolate vaccine effectiveness in high-grade cases. Among women who received full vaccination (compared with no vaccination), the adjusted OR for high-grade cervical abnormalities was 0.50 (95% CI 0.30–0.85), which translates into a vaccine effectiveness of 50% (95% CI 15%–70%). The adjusted odds of high-grade cervical abnormalities with at least 1 dose of vaccination compared with no vaccination were lower when the control was redefined as both normal cytology outcomes and low-grade cervical abnormalities (adjusted OR 0.46, 95% CI 0.28–0.75).
A separate analysis of Alberta’s cervical screening outcomes found that the PPV of Pap tests processed at SurePath was higher than the PPV of those processed at ThinPrep for women aged 18–21 (57.3% v. 46.6%, p = 0.02), and the difference decreased but persisted in other age groups.
Interpretation
Eight years after a school-based HPV vaccination was initiated in Alberta, 3-dose HPV vaccination has demonstrated early benefits, particularly against high-grade cervical abnormalities, which are more likely to progress to cervical cancer.1
The HPV vaccine effectiveness we report is comparable to that of other observational studies with pathology outcomes,6,8 but greater than an Australian study’s age-adjusted vaccine effectiveness against high-grade cytologic abnormalities with complete vaccination (39%, 95% CI 22%–52%).7 The substantial vaccine effectiveness found in the current study may be attributable to our study population of only young women, likely to be HPV-naive when vaccinated.6 Furthermore, North American populations have a higher proportion of vaccine-targeted HPV types 16 and 18 than other regions.25
Effective HPV vaccination will disrupt the balance of harms and benefits of cervical cancer screening.26 The reduced prevalence of HPV-related cervical abnormalities will reduce the PPV of the screening test.27 The reduced prevalence also means that there will be fewer referrals to colposcopy, which is associated with discomfort and potential obstetric complications.16,28 Thus, with population-based HPV vaccination, guidelines for cervical cancer screening may need to include a later age for screening initiation and/or a longer interval between screenings.
Optimal cervical cancer prevention requires both effective HPV vaccination and screening.4 Screening may prevent cervical cancer for both unvaccinated and vaccinated women, considering that not all cancers and tumours are associated with the HPV strains included in the quadrivalent vaccine.27,29 Thus, it is concerning that unvaccinated women are less likely to be screened (Table 1). The interaction between vaccination and screening behaviour leads to the population becoming stratified into 3 distinct categories for cancer risk: low-risk groups are both vaccinated and being screened; medium-risk groups are either vaccinated or followed up appropriately with screening; and high-risk groups are neither vaccinated nor screened. Emergence of the high-risk group, more vulnerable to cervical cancer, means that vaccination and screening programs should work together to identify, monitor and intervene with individuals marginalized by both programs. The collaboration can extend to the low-risk group to avoid overscreening.
A topical issue in Canada and elsewhere is the effectiveness of 2-dose HPV vaccination; the National Advisory Committee on Immunization (NACI) in Canada allows a 2-dose HPV vaccination schedule.30 The lack of effectiveness of the 2-dose schedule found in this study might be from secondary vaccine failure, as the separation between last vaccination and Pap test was longer than 36 months known for immunogenicity of the two-dose vaccination.30,31 Furthermore, median separation between first and second doses in our study was shorter than the recommended 6 months.14 A 6-month period is required for affinity maturation of memory B cells for lasting immunity.32 The efficacy of the 2-dose schedule in trials has not been reproduced in observational studies that did not have the recommended dose spacing.6,8,30 An Australian study with proper spacing found that the 2-dose schedule had substantial vaccine effectiveness in cytology outcomes but not in pathology outcomes.33 At this point, a policy change to a 2-dose schedule for HPV vaccination should be done with caution given that evidence for vaccine effectiveness is still inconclusive.
There was a significant difference in the proportion of cervical abnormalities between the 2 laboratories in Alberta. The laboratories are different in the geographical distribution of their clients. In Alberta, prevalence of sexually transmitted infections is different along the north–south geographical division, although patterns are mixed; the incidence rate of chlamydia is higher in the northern half whereas the incidence of syphilis is higher in the southern half.34 The finding merits further investigation, and the ACCSP has plans to investigate these laboratory differences in Alberta.
Strengths and limitations
A strength of our study is the use of deterministically linked population-based surveillance databases for both cervical cancer screening and HPV vaccination. This approach reduces selection bias, as well as misclassification risk from probabilistic linkage. In addition, our population is restricted to the cohort targeted by the school vaccination program. Thus, confounding that may occur from prevaccination HPV infection if age ranges are too wide is reduced.
A limitation of the study is that we did not use pathology outcomes. The accuracy of cytology compared with pathology varies, especially for low-grade abnormalities,35 which accounted for most of our study’s cases. Because most of our study population are not candidates for colposcopy owing to young age,36 we did not have large enough and representative biopsy outcomes for the study purpose. Despite this limitation, cytology outcomes are worthwhile to study given that they have been used to determine the outcomes of screening programs.26
Conclusion
Quadrivalent HPV vaccination significantly reduced high-grade cervical abnormalities but required 3 doses. Vaccination against HPV was associated with screening uptake. Our study provides early evidence for the effectiveness of HPV vaccination. A cohort study using a population with both appropriate vaccination and screening age may validate our study’s finding of an interaction between HPV vaccination and screening uptake. When outcomes from areas implementing 2-dose HPV programs become available, the effectiveness of a 2-dose schedule will be clearer. Evidence from this study and future studies can be used to improve integration of HPV vaccination and screening programs for preventing cervical cancer.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Huiming Yang, Lawrence Svenson and Gordon Kliewer were involved in the study conception. Jong Kim, Christopher Bell, Gordon Kliewer, Linan Xu, Maggie Sun and Maria McInerney were involved in the study design, the acquisition, analysis and interpretation of data. Jong Kim, Christopher Bell and Maria McInerney drafted the manuscript. All of the authors revised the manuscript, approved the final version to be published and agreed to act as guarantors of the work.
- Received July 4, 2016.
- Accepted March 22, 2016.