Abstract
Background: Sophisticated approaches are needed to improve the quality of care for elderly people living in residential care facilities. We determined the effects of multidisciplinary integrated care on the quality of care and quality of life for elderly people in residential care facilities.
Methods: We performed a cluster randomized controlled trial involving 10 residential care facilities in the Netherlands that included 340 participating residents with physical or cognitive disabilities. Five of the facilities applied multidisciplinary integrated care, and five provided usual care. The intervention, inspired by the disease management model, consisted of a geriatric assessment of functional health every three months. The assessment included use of the Long-term Care Facility version of the Resident Assessment Instrument by trained nurse-assistants to guide the design of an individualized care plan; discussion of outcomes and care priorities with the family physician, the resident and his or her family; and monthly multidisciplinary meetings with the nurse-assistant, family physician, psychologist and geriatrician to discuss residents with complex needs. The primary outcome was the sum score of 32 risk-adjusted quality-of-care indicators.
Results: Compared with the facilities that provided usual care, the intervention facilities had a significantly higher sum score of the 32 quality-of-care indicators (mean difference − 6.7, p = 0.009; a medium effect size of 0.72). They also had significantly higher scores for 11 of the 32 indicators of good care in the areas of communication, delirium, behaviour, continence, pain and use of antipsychotic agents.
Interpretation: Multidisciplinary integrated care resulted in improved quality of care for elderly people in residential care facilities compared with usual care.
Trial registration: www.controlled-trials.com trial register no. ISRCTN11076857.
See related commentary by Stadnyk and colleagues at www.cmaj.ca/lookup/doi/10.1503/cmaj.110789.
The quality of care provided in residential care facilities is under pressure worldwide.1 Facilities are frequently understaffed, and the complexity of care needed by residents increases while expertise of staff does not necessarily keep pace.2,3 Although most care organizations want to innovate and improve quality of care, many lack expertise or financial resources needed to do so.4,5 Family physicians are responsible for medical care in residential care facilities in the Netherlands. However, they do not regard themselves as suited for systematic management of chronic diseases and disabilities associated with frail health.6
About 10% of elderly people aged 75 or older in the Netherlands live in residential care facilities.7,8 These facilities were established to offer sheltered living for elderly people who are disabled but still relatively healthy. Because of the growing elderly population, the characteristics of elderly people living in residential care facilities have become more comparable to those of people in nursing homes, who need complex care. Residential care facilities in the Netherlands are comparable to residential care facilities in Canada, are publicly funded and are subject to government inspection and approval. Over 70% of the residents need professional care, such as assistance with activities of daily living, nursing care (e.g., medication, wound care) and housekeeping. They have multiple chronic diseases and associated disabilities.9–12
Effective interventions for chronic illnesses generally rely on a multidisciplinary team approach. The elements of this approach include structured geriatric assessment, protocol-based regulation of medications, support for self-reliance and intensive follow-up. The closely related disease management model comprises coordination of care, steering of the care process and patient empowerment.13 This model is strongly recommended by Bodenheimer and colleagues to improve the health and quality of life of chronically ill patients.14 However, no studies have as yet been undertaken to evaluate the effects of disease management on functional health and quality of care for elderly people in residential care facilities who have physical or cognitive disabilities.
We developed an approach to multidisciplinary integrated care inspired by the disease management model. The objective of our study was to determine the effects of multidisciplinary integrated care on quality of care and quality of life for elderly people in residential care facilities.
Methods
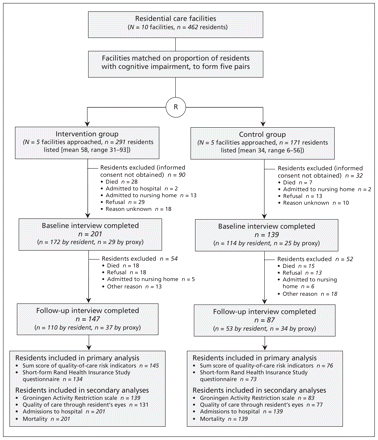
The study was approved by the ethics committee of the VU University Medical Center in Amsterdam, the Netherlands. All participating residents or their proxies provided written informed consent. Figure 1 shows the flow of participants through the study.
Flow of completed interviews of participants through the trial. Because of missing data, the numbers of residents included in the primary and secondary analyses differed from the numbers interviewed. R = randomization.
Study design and participants
After a pilot study in one residential care facility, a cluster randomized controlled clinical trial was set up involving 10 facilities belonging to the umbrella care organization Omring, a large home care and long-term care provider in the Netherlands. The pilot facility was excluded from the trial. Randomization was carried out at the facility level; five facilities were assigned to the intervention group and the other five to the usual care group.
A total of 462 residents from the 10 facilities were recruited from December 2006 until December 2007. The average number of residents in each facility was 46, and staff included nurse-assistants and a house manager. All residents were listed in a primary care practice whose physician was responsible for their medical care. Residents who were terminally ill (as determined by staff or family physician) were excluded from the study.
Participating residents in each facility were visited by trained, blinded interviewers at baseline and at six months. If the resident was unable to understand the questions, a close family member was identified by staff and asked to act as proxy. The interview consisted of a computerized assessment of functional health, activities of daily living, depression, cognition, satisfaction with care, and use of medications. Proxies completed the interview except for the cognitive assessment, which was replaced by a short form of the Informant Questionnaire on Cognitive Decline in the Elderly.15
The duration of the trial was relatively short because of a high risk for dropout owing to the extreme vulnerability of residents and because the umbrella care organization intended to implement the care model in the control facilities as well. A detailed description of the design was reported earlier.16
Randomization
Randomization was carried out on facilities after matching for percentage of cognitively impaired residents, based on the assumption that a high percentage of such residents would affect care-related needs and services. In the matching procedure, the two facilities with the highest percentage of cognitively impaired residents were matched, and so on. Randomization was carried out using the first column from Pocock’s random numbers table.17
Intervention
By adapting the principles of disease management, we introduced the concept of multidisciplinary integrated care. This concept focused on identification and monitoring of the functional disabilities caused by chronic diseases. Its three basic elements correspond to those of the disease management model: monitoring of disabilities, coordination of care and empowerment.13 The third element is normally applied to patients only. However, we wanted to empower nurse-assistants in relation to monitoring and coordination of care, given that they provided all basic nursing care.
The model of multidisciplinary integrated care used in our study comprised five elements.16 First, a geriatric multidimensional assessment of all residents was conducted every three months. The Web-based Long-term Care Facility version 9.0 of the Resident Assessment Instrument was used for this purpose.18 The identified problem areas guide the design of an individualized care plan that is intended to improve or maintain functional health status. Second, the care plan was discussed with the resident, the resident’s family and the family physician, and adapted to personal wishes. Third, residents with complex care needs were scheduled at least twice a year for a multidisciplinary meeting. Fourth, consultation with a geriatrician or psychologist was optional for the frailest residents with complex health care problems. Fifth, data from the Web-based Resident Assessment Instrument was used to provide an overview every three months of 32 risk-adjusted quality-of-care indicators. These indicators were compared with benchmark values derived from data on all residents of residential facilities in the Netherlands obtained using this instrument.19,20 Further details of the model of multidisciplinary integrated care are found in Appendix 1 (available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.101498/-/DC1).
Usual care
For facilities assigned to usual care, the family physician was responsible for medical care and offered it on request. There was neither coordination nor structured planning of care. Multidisciplinary meetings were mostly not attended by the family physicians.
Outcome measures
For the purpose of the evaluation, experienced, specially trained, blinded and supervised interviewers independently assessed the residents at baseline and six months later. The interviewers’ assessments were supplemented by systematic observations by staff and extraction of data from residents’ medical records (e.g., actual medication regimen).
Primary outcome measures
The first primary outcome was the sum score of the 32 risk-adjusted quality-of-care indicators, which were developed by Morris21 and showed good validity and reliability. Appendix 2 (available at www.cmaj.ca/cgi/content/full/cmaj.101498/DC1) shows the definitions of the quality-of-care indicators, including their numerator and denominators. The quality-of-care indicators were originally based on observations recorded in the Long-term Care Facility assessment form. We incorporated the itemized observations needed to calculate these indicators in the assessments performed by our independently trained interviewers. Interrater reliability of the quality-of-care indicators between interviewers and nurse-assistants in the intervention facilities was satisfactory (mean intra-cluster correlation single measure 0.74). The sum score of the quality-of-care indicators was determined by the number of indicators per resident divided by the number of indicators applied to a resident. Indicators were calculated using mixed linear hierarchical models.
Four of the 32 quality-of-care indicators (behaviour problem, bladder or bowel incontinence, pressure ulcer and use of antipsychotics) applied both to the group of residents as a whole and to high- and low-risk groups. We therefore calculated two sum scores, one for all 32 indicators and one for the 24 whole-group indicators after exclusion of the 8 that were broken down to apply to high- and low-risk groups. Of these 24 whole-group indicators, on average 21 (standard deviation [SD] 6.6) applied to the residents. Of all 32 indicators, on average 22 (SD 6.1) applied to the residents. The relatively lower number of applicable indicators among all 32 indicators is due to the inclusion of the breakdown indicators, which applied to a maximum of 50% of the residents. The Cronbach’s α of the sum score of the 24 whole-group indicators was 0.62.
For the second primary outcome, health-related quality of life was measured using a short-form 12-item version of the Rand Health Insurance Study questionnaire.22 Its properties were satisfactory when used by proxies, which was important in our study because of the percentage of cognitively impaired residents (58.2%).23 We also calculated the number of quality-adjusted life-years using the algorithm of Hatoum and colleagues.24
Secondary outcome measures
The secondary outcome measures comprised the 32 individual risk-adjusted quality-of-care indicators (described in Appendix 2); activities of daily living, as measured by the Groningen Activity Restriction Scale designed for elderly respondents and validated by Kempen and coauthors;25 quality of care from the residents’ perspective, as measured by a short (16-item) version of the QUOTE–Elderly instrument (Quality of Care from the Perspective of the Elderly);26 hospital admissions recorded at the (single) local hospital, which covered more than 95% of all admissions;16 and mortality, as recorded by the interviewers or staff and cross-checked by the administration of the municipality.
Process outcomes
Process outcomes comprised the percentage of residents with completed assessments; the number of multidisciplinary meetings held, based on minutes of the meetings; the numbers of agreed-on medical, nursing and social actions, based on content analysis of care plans; and opinions of participating professionals regarding the intervention protocol, as obtained by interviews with staff and family physicians.
Statistical analysis
Sample-size calculations
Sample-size calculations were based on the expected effects of the intervention on quality of care and functional health using an α level of 0.05, a power of 80%, a dropout rate of 15% and an anticipated intracluster correlation of 0.05, based on Adams and colleagues,27 across the residential facilities. To detect a fair benefit (i.e., Cohen’s d effect size of 0.5), we estimated that the sample should include at least 82 residents in each of the two study groups.28
Effect analyses
Effect analyses were performed according to both intention-to-treat and per-protocol principles. We accepted that the protocol was adhered to when the first two (obligatory) steps of the intervention were performed. We compared differences in the outcome measures over six months between the intervention and control groups using multivariable techniques. We applied mixed models for the continuous outcomes, using an unstructured covariance matrix for the longitudinal data. For the dichotomous outcomes, we applied generalized estimating equations using a logit link and an exchangeable working correlation.
In all effect analyses, we adjusted for baseline imbalance (e.g., by age, sex, cognitive impairment, depression, disability and interview by proxy). The analyses were done with multilevel specification if these variables showed significantly higher log-likelihood estimates. Because of our cluster randomized design (with randomization at the facility level), results of multilevel and “simple” analyses were compared for all outcomes. If higher-order clustering effects were present, outcomes of the multilevel analyses were presented; if clustering was negligible, results of “simple” analyses were presented.
The intracluster coefficient across facilities was estimated by exchangeable working correlation for all individual (dichotomous) quality-of-care indicators. In all outcomes with pre–post measurements, the effect of interest was the group × time (pre–post) interaction effect. A p value of 0.05 was considered to be significant.
Process analyses
We evaluated the extent to which the intervention program was performed according to protocol, the nature of the recommendations of the multidisciplinary meeting and the judgments of family physicians and staff about the intervention program.
Results
Sample and facility characteristics
Baseline characteristics of the residents and facilities are described in Table 1. There was a small imbalance between the intervention group and the usual care group in the numbers of residents with cognitive impairment and in the numbers with clinical depression. The trial ended up imbalanced because one control facility did not accept new entries as a result of an upcoming move to another location, and because a second control facility was in the middle of moving to a new building and could therefore recruit few residents for the study. Analyses without these facilities did not change the results.
Baseline characteristics of the 10 residential care facilities and the 340 participating residents
Primary outcomes
Compared with residential care facilities that provided usual care, the intervention facilities had a significantly higher sum score of the 32 risk-adjusted quality-of-care indicators (mean difference −6.7 (95% confidence interval [CI] −8.69 to −4.71, p = 0.009; Cohen d effect size 0.72) (Table 2). Self-reported quality of life did not differ between residents of control and intervention facilities (Table 3).
Risk-adjusted indicators of quality of care for elderly people in intervention and control residential care facilities during the six-month study period
Health-related outcomes and residents’ opinions of quality of care
Secondary outcomes
The intervention facilities had higher scores than the control facilities for 30 of the 32 risk-adjusted indicators of quality of care; the scores for 11 of these 30 indicators had increased significantly (Table 2). In the intention-to-treat analyses, no differences in disability or quality of care as seen through residents’ eyes were found between the two groups of facilities (Table 3). In the per-protocol analysis, residents in the intervention facilities tended to be more positive about the quality of care over time than residents in the usual care facilities (difference 1.8, p = 0.072). The per-protocol analyses showed that fewer residents died in the intervention group than in the control group (intervention 10/112, control 25/139; odds ratio 2.15, 95% CI 1.06–4.38; p = 0.035).
Process of care
The first step of the protocol — assessment with the Long-term Care Facility version of the Resident Assessment Instrument — was completed for 55.2% of the residents in the intervention facilities. This proportion was less than we had aimed for and was partly a result of implementation delay.
Forty multidisciplinary meetings were held in the intervention facilities during the study period, and the outcomes of assessment of 93 residents included in the study were discussed (Table 4). The primary care physician was present at 90% of the multidisciplinary meetings, and the geriatrician at 75%. By contrast, only 25% of the multidisciplinary meetings in the control facilities were visited by the primary care physician. The number of recommended actions per resident was 3.67 in the intervention facility meetings and 2.26 in the control facility meetings.
Characteristics and outcomes of multidisciplinary meetings held during the six-month study period
The expertise of staff was felt by 52.9% of staff and 54.5% of the family physicians to have increased after introduction of the care model. The same percentage of staff and 63.6% of family physicians felt that they had more knowledge about residents’ health. In addition, 58.8% of staff and 81.8% of the family physicians felt that their cooperation had improved. About 55% of the family physicians considered quality of care to have improved; 73% acknowledged the need for a new care model.
Ancillary analyses
We did not find effect modification of the outcomes by age, sex or baseline disability.
Interpretation
Compared with usual care, our model of multidisciplinary integrated care resulted in substantially higher quality of care for elderly people in residential care facilities. Functional ability, number of hospital admissions and health-related quality of life remained comparable between the two groups. According to the per-protocol analyses, mortality was lower in the intervention facilities and residents in the intervention facilities were more positive about their quality of care. Owing to the short intervention period (six months), the full protocol was applied to less than half of the residents in the intervention facilities. The training and empowerment of nurse-assistants, which was completed for all intervention facilities, together with monitoring using the geriatric assessment instrument, were likely to be the most important ingredients for improvement of the quality of care.
Earlier studies have reflected elements of our intervention. For example, positive health effects on residents have been reported as a result of interdisciplinary geriatric primary care in American facilities.29 Integrated and home-based geriatric care management was reported to improve quality of care and reduce use of acute care services in a high-risk group of low-income elderly people living at home.30,31 Use of the Home Care version of the Resident Assessment Instrument in primary care health centres in Hong Kong resulted in improvement in 2 of 13 functional outcomes.32 Modest positive effects on well-being and on deterioration of functional skills were found in a multidisciplinary program in vulnerable older people living at home.33
Limitations
Our study was limited by the fact that our population was frail and comprised a high percentage of cognitively impaired residents. As a result, a portion of the data was collected from interviews with proxies. The judgments of proxies may have differed from the residents’ judgments. Therefore, we adjusted for proxy interview and cognitive status in our analyses. The cluster randomization produced an imbalance between the intervention and control facilties in the number of participating residents and in some of the functional characteristics of the residents at baseline. Although we adjusted for the imbalance in functional characteristics, imbalance in the number of participating residents may have led to underpowered results.
Variation across the intervention facilities in the application of the complete protocol (3%–66%) was another limitation. This variation can be explained by financial and administrative issues during the study period. The financial obligations for residential care facilities resulting from a new national funding system for residential care of elderly people caused uncertainty about job continuation, high turnover of managers and new priorities at the facilities in our study. Despite this limitation, the improvement in quality of care at the facilities in our study was substantial.
Conclusion
Our model of multidisciplinary integrated care resulted in improved quality of care for elderly people in residential care facilities compared with usual care. The results of this study are applicable to elderly people in such settings as residential care facilities and nursing homes, and even elderly people living in the community. In primary care settings, it may be beneficial to have a model to monitor elderly people and those with chronic diseases, to prevent functional decline and admission to hospital for acute care. It is also important to have an instrument that not only delivers output on the patient level but also on the management level, to facilitate monitoring of quality of care by managers in a sector of health care that is under enormous societal pressure to improve its performance.
Footnotes
-
Competing interests: None declared.
-
This article has been peer reviewed.
-
Contributors: Marijke Boorsma, Hein van Hout and Giel Nijpels were involved in the conception and design of the study. Marijke Boorsma and Dinnus Frijters were involved in the acquisition of the data. Marijke Boorsma, Hein van Hout, Dirk Knol and Miel Ribbe were involved in the analysis and interpretation of the data. Marijke Boorsma and Hein van Hout were involved in the drafting of the article. All of the authors were involved in the critical revision of the manuscript for important intellectual content and approved the final version submitted for publication.
-
Funding: This study was funded by the Netherlands Organization for Health Research and Development (ZonMw grant no. 945-05-030). The funding organization had no role in the design and conduct of the study; the collection, management, analysis and interpretation of the data, or the preparation, review and approval of the manuscript.