Scientists have predicted that extremes in climate are likely to increase in frequency and severity. 1 These changes may have a direct impact on population health, as heat waves can exceed the physiological adaptive capacity of vulnerable population groups. Individuals over the age of 60 years are consistently the most vulnerable, 2–4 with 82%–92% of excess mortality occurring in this group. 5 Risks for heat-related illness or injuries are compounded for people with obesity, 6,7 cardiovascular disease, 8–10 respiratory disease 8–10 and diabetes mellitus. 4,8,9 These conditions decrease the body’s ability to adapt to changes in environmental conditions. 11 When people must perform physical work in the heat, the occurrence of heat-related morbidity and mortality is likely to be more frequent. 12 Although these trends in heat-related morbidity and mortality are evident, there has been little research to explain the causes of increased susceptibility within vulnerable populations.
In this review, we describe the effects of heat on human physiology and the factors that increase the risk of heat stress. The methods used in preparing this review are summarized in Box 1 and are described in greater detail in Appendix 1 (available at www.cmaj.ca/cgi/content/full/cmaj.081050/DC1).
Box 1: Review methods
Thermoregulatory responses in healthy people
In healthy people, the body regulates its core temperature to maintain a near-constant level (about 37ºC), irrespective of environmental conditions. To do this, the thermoregulatory system adjusts a variety of physiological mechanisms to attain a balance between the heat produced within the body and the heat lost to the environment, through a combination of dry heat exchange and evaporative heat loss. At room temperature, resting metabolic heat production is balanced primarily by dry heat loss through conduction, convection and radiation. 13
Heat balance is easily disturbed during exposure to a warmer environment and with changes in metabolic heat production (due to physical activity). As the ambient air temperature increases, the capacity for dry heat exchange is reduced because of a reduction in the temperature gradient between the skin surface and the ambient air. 13 When the environment is warmer than the skin, the body gains heat through dry heat exchange, which increases the requirements for sweating and circulatory responses to achieve a given rate of heat dissipation. During physical activity, the increase in metabolic rate above resting levels increases the rate at which heat must be dissipated to the environment to prevent the body core temperature from rising to a dangerous level. The extent to which body core temperature increases at steady state is largely independent of the ambient air temperature (within the measured range of 5°C–36°C) and is proportional to the metabolic rate. 14 With metabolic or environmental heat load or both, the increase in sweat rate and skin blood flow is proportional to the amount of evaporative heat loss required for heat balance. 13 Under circumstances in which changes in sweating and skin blood flow cannot facilitate a sufficiently high rate of heat loss (e.g., intense physical activity in hot or humid conditions, impairment of normal thermoregulatory function), body core temperature rises continuously; left unchecked, this rise in body core temperature may lead to heat illness and eventually death.
Exposure to the combination of external heat stress and metabolically generated heat can lead to heat-related disorders. The prevalence of heat stress symptoms increases in direct proportion to the elevation of body core temperature. The major heat-related disorders — heat cramps, heat exhaustion and heatstroke — involve various degrees of thermoregulatory failure, which occurs when a person is exposed to excessive heat or elevations in body core temperature over a prolonged period.
Risk factors for heat stress
Age
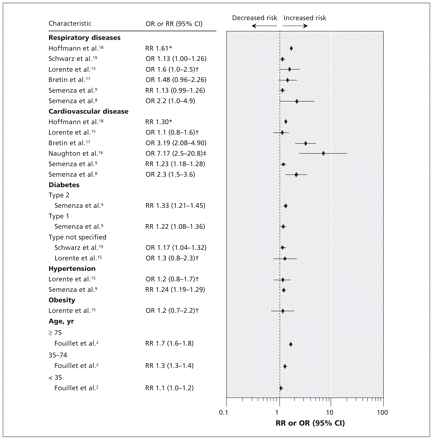
Observational studies have shown that people aged 60 years and older are among the worst affected by extreme heat, 3–5 with those living in institutions, confined to bed or living alone having the highest rates of illness, injury and death. 5,8,15–17 In their ecological time-series study, Fouillet and associates 2 showed that during the 2003 heat wave in Europe, mortality ratios (ratios of observed deaths to expected deaths) in France increased continuously with age, from 1.3 for people 35–74 years of age to more than 1.7 for those over the age of 75 (Figure 1). Although the greater prevalence of comorbidities and medication use in this population may be responsible for some of the heat-related deaths, laboratory-based physiological studies have indicated that the ability to sense heat 20 and to manifest appropriate behavioural (especially fluid intake) 21–26 and physiological (e.g., blood distribution, sweating) responses 27–33 during exposure to heat may be compromised in otherwise healthy older individuals.
Figure 1: Relative risks (RRs) or odds ratios (ORs) for age and various health conditions. RRs compare hospital admission 9 or mortality 2,18 during a heat wave with mortality (or hospital admission) observed during comparison dates without heat wave. ORs compare cases who died during a heat wave with controls in the same geographic area who did not die. Values greater than 1.0 indicate a greater risk of heat-related death or hospital admission. *95% confidence intervals (CIs) were not provided in this study. †Values were adjusted for sex and autonomy (ability to get out of bed, wash and use bathroom without help). ‡Values were adjusted for neighbourhood and age.
The ability to physiologically maintain body core temperature during heat stress becomes compromised with age. 27 This decrease in thermoregulatory ability can be attributed to a combination of factors, including changes in sweating, 28–30,34 blood flow to the skin 30,31,34,35 and cardiovascular function. 32 The problem can be exacerbated by the decreases in overall physical fitness and increases in body adiposity that may accompany aging. 36 Experts have suggested that, in combination, these age-related changes in thermoregulatory and cardiovascular function can decrease the body’s ability to maintain body core temperature at safe levels, especially during extended exposure to heat or to physical activity in the heat. 37
The results of a recent physiological study 20 suggested that older people are less sensitive to heat exposure and take longer to respond to temperature changes than their younger counterparts. The age-related decrease in sweat rate does not appear to be due to a decrease in the number of activated sweat glands, 28,29 but rather to a reduction in the amount of sweat produced per gland, as shown by studies in which the sweat glands were stimulated pharmacologically. 28,29,33 Older individuals also experience reductions in evaporative heat loss and overall sweat rate during physical activity. 27
Laboratory-based physiology studies have shown that the reduced sweat output is accompanied by a decrease in skin blood flow during exposure to heat. 30,31,34 Although the researchers observed no age-related differences in the onset of skin vasodilation, reflex increases in skin blood flow in response to increasing core temperature were attenuated in aged skin during passive heat exposure, independent of the level of acclimation. 30 Age-related decreases in skin blood flow during passive heat exposure were associated with reduced cardiac output (despite a similar heart rate response) and less redistribution of blood flow from renal and splanchnic circulations. 32 Similar age-related reductions in skin blood flow have been observed during exercise in the heat. 31
Older adults may also have a decreased ability to sense and adapt to dehydration. In physiology studies in which dehydration was induced through heat exposure alone, 24 through physical activity in the heat 25,26 or through hypertonic saline infusion, 23 healthy older individuals exhibited lower subjective levels of thirst, decreased plasma volume and reduced water intake while dehydrated relative to younger counterparts. Recovery from dehydration is prolonged in older adults, which may exacerbate their risk of heat-related injuries during extended periods of heat exposure, such as heat waves. 21
In spite of the apparent thermoregulatory impairments, there is evidence showing that older adults are capable of adapting. 30,33 Both passive heat exposure and mild to moderate exercise in a very hot environment (i.e., above 40°C) over an 8- to 10-day period resulted in adaptation in older people. 30,33 Physiological changes included lower skin and rectal temperatures, increased output of sweat, decreased concentration of sodium in the sweat and a reduced temperature threshold for the onset of vasodilation and sweating. 30,33 Despite these adaptations, the responses to heat were attenuated after repeated exposure to heat, relative to younger participants undergoing the same acclimation protocol. 30,33 Whereas physical activity in the heat led to increased thirst, augmented fluid intake and greater plasma volume in young individuals, these adaptations may be attenuated in older individuals. 21,22
Obesity
Fatal heatstroke occurs 3.5 times more frequently in adults with overweight and obesity than in individuals of average body weight. 38 Whether this is due to impairments in thermoregulation remains essentially unknown, as very few studies have examined heat exposure in obese individuals. Nonetheless, some evidence indicates that both heat-sensing and heat-dissipating abilities may be impaired in this population.
Obese individuals may have lower thermal sensitivity to heat stimuli. 39 The mechanism for this is thought to be related to underlying small-fibre neuropathies, which may in fact be common in people with obesity, even in the absence of clinical sensory impairment. 39 It is unclear, however, if a reduction in thermal sensitivity is associated with a reduction in heat loss effector responses.
A lower capacity for heat dissipation may be responsible for the greater frequency of heat-related injury and illness in this population. 38 An obese person has a smaller ratio of body surface area to body mass for effective sweat evaporation than a leaner person of the same height, because the greater body mass is not proportional to any difference in the size of the interface for heat exchange with the environment. Furthermore, excess body fat is a liability during exposure to hot environments. The specific heat capacity of adipose tissue is less than that of fat-free mass (2.97 kJ·g−1·°C−1 v. 3.64 kJ·g−1·°C−1), which means that a given amount of heat storage per unit body mass will cause a greater increase in average tissue temperature in a person with higher body-fat mass. In addition, the thermal conductivity of fatty tissue is lower than that of other tissues in the body. The convective transfer of heat from the body core to the outer shell (the skin) via the circulation is theoretically independent of body fat, since the peripheral blood vessels are located closer to the surface than the subcutaneous fat layers; however, levels of skin blood flow for given elevations in core temperature have been shown to be lower in people with obesity than in those who are not obese. 40 A greater gradient between core and skin temperature develops quickly in a person with obesity, which increases the drive for conductive heat transfer from the body core. However, a thicker subcutaneous layer of adipose tissue restricts this conductive heat transfer and further escalates the risk of heat stress.
The extra weight that obese individuals carry also increases the metabolic cost of weight-bearing activities, which elevates the rate of heat production. As shown by Bar-Or and colleagues, 6 people with obesity exhibit greater elevation in core temperature than lean individuals for the same absolute workload. This response is independent of ambient temperature and occurs in parallel with elevated sweat rate and greater cardiovascular strain. 6 The reflex increase in skin blood flow in response to elevated core temperature may also be impaired in obese individuals. 40
Hypertension
Hypertension is characterized by elevation of peripheral resistance and is accompanied by a variety of peripheral circulatory changes, including hypertrophy of the vascular smooth muscle 41 and vascular rarefaction. 42 Each of these changes could lead to impairments in the control of blood flow in the skin 43 and consequently weaken core temperature regulation. During exercise-induced heat stress, people with hypertension experience an elevated blood pressure response and possibly greater thermal strain than people with normotension. 44 In earlier studies, Kenney and collaborators 44,45 found that the increases in forearm blood flow during exercise-induced hyperthermia, which are primarily confined to the skin, 46 were markedly reduced in people with hypertension relative to people with normotension. This effect could result in a reduction in heat transfer from the body core to the skin, which would increase the potential for heat illness. Whether exercise performed under hot ambient conditions exacerbates this effect remains unclear. It should be noted that the use of antihypertensive medications (diuretics, vasodilators, β-blockers) may significantly reduce heat tolerance when combined with exercise and warm ambient conditions. 47,48
The clinical impact of cardiovascular responses during heat exposure in people with hypertension is unknown. Some studies have suggested that thermoregulatory responses to passive heat exposure may in fact be unaltered in people with hypertension. 49,50 However, the heat exposure in those studies might not have been of sufficient intensity to capture the results of impaired heat loss responses.
Diabetes mellitus
Epidemiologic data indicate that individuals with diabetes have significantly higher rates of heat illness and death during heat waves than the general population, with excess in-patient admissions being 30% more numerous (95% confidence interval 4.6%–55.9%, p = 0.033; percentages calculated from raw data in published table) during recorded heat waves 9 (Figure 1). Diabetes is associated with several metabolic, cardiovascular and neurologic dysfunctions, which may also play roles in impairing thermoregulatory mechanisms during heat exposure.
In both type 1 diabetes 51 and type 2 diabetes, 51–53 the ability of the blood vessels in the skin to dilate may be impaired, which could decrease the amount of blood being brought to the skin’s surface to dissipate heat. When skin blood flow responses were compared in healthy people and people with type 1 and type 2 diabetes (age-matched, ranging from age 18 to 71 years), vascular reactivity was decreased during 5 minutes of heat exposure among those with diabetes. 51 Another study showed that the internal temperature at which vasodilation took place during passive whole-body heat exposure was higher for people with type 2 diabetes than for healthy individuals. 53 The delayed onset of skin vasodilation was due to a delay in the active vasodilatory response, not to an increase in vasoconstrictor activity.
Poor glucose control and the presence of neuropathy may affect sweating responses in individuals with type 2 diabetes. 54,55 Sweating response during passive heating was impaired in people with type 2 diabetes, especially in the distal regions. 54 In a separate study, the sweat rate was reduced, especially in the limbs, among people with type 2 diabetes, both at rest and during exercise in the heat. 52 The lower sweat rate was thought to be associated with diabetes-related peripheral neuropathy and autonomic dysfunction. Sweating responses to pharmacologic stimuli are maintained in individuals with non-neuropathic diabetes but impaired in those with severe neuropathy. 55 We found no studies that directly measured the impact of these impairments on core temperature during physical exposure to heat or exercise.
Vascular impairments in type 1 diabetes occur with increasing disease duration and greater degrees of hyperglycemia. 56,57 Using postocclusive hyperemia as a means of observing maximal blood flow, 2 groups of researchers found that participants with diabetes and poor glucose control (i.e., average glycated hemoglobin > 7.5%) had poorer blood flow to the limbs than both nondiabetic controls and individuals with diabetes who had good glucose control (i.e., average glycated hemoglobin < 7.5%). 57,58 Similar results were obtained when local heat applied to the foot was used as a stimulus, the participants with diabetes (average glycated hemoglobin 8.7%) having lower microvascular dilatation and lower resulting skin blood flow than their nondiabetic counterparts. 56 Some of these responses may be altered in the presence of high insulin levels. 59 Whether these decreases in local skin blood flow affect heat delivery to the skin surface and the resultant core temperature response is unknown.
Sweat response can be altered in type 1 diabetes as a result of neuropathy. In a longitudinal study of patients with newly diagnosed type 1 diabetes, the sweat response to a pharmacologic stimulus (acetylcholine) was elevated in the early stages of the disease but decreased with increasing duration of the disease, 55,60 which would potentially affect the body’s ability to maintain core temperature. Differing degrees of sweating impairment during whole-body heat exposure (44°C–50°C) have been found in people with type 1 diabetes, the loss of sweating response being related to the presence and severity of neuropathy. 54 There is no direct evidence to indicate how these altered sweat responses affect changes in core temperature during exposure to heat in this population.
Apart from the major cardiovascular and thermoregulatory responses that take place during acute exercise and heat stress, a number of metabolic alterations associated with diabetes may reduce heat tolerance and affect exercise performance in the heat. Early physiological studies suggested that insulin action in type 1 diabetes may be increased by exposure to high temperatures, increasing the risk of hypoglycemia. 61,62 We found no studies on this topic examining patients treated with newer forms of insulin or newer insulin delivery systems (i.e., insulin pumps).
Cardiovascular disease
Cardiovascular disease encompasses heart and circulatory ailments, including coronary and valvular heart disease, chronic heart failure, cardiomyopathy, congenital heart defects, and cerebrovascular and peripheral vascular disease. 63 Ecological time-series data from the 2003 European heat wave showed that mortality rates among people with cardiovascular disease were 30% greater during the heat wave than during comparison periods 18 (Figure 1). Individuals with pre-existing cardiac dysfunction have a reduced ability to increase their cardiac output sufficiently to maintain adequate skin blood flow when core temperature is elevated. 64,65 If there is long-term exposure to an extreme heat event, stress on the heart and other organs can be exacerbated by dehydration as body core temperature increases. 66 In healthy individuals, even relatively small decrements in hydration status (1% of body weight deficit) can impair the cardiovascular and thermoregulatory responses. 67 Although no studies have directly measured the effect of these impairments on core temperature regulation in individuals with cardiovascular disease, increases in blood viscosity (hemoconcentration) due to dehydration can impose a substantial burden on the cardiovascular system. 64,66
Heat-related inflammation and coagulation may have contributed to the near doubling of deaths due to cerebral and coronary thrombosis during a London heat wave. 66 When healthy individuals were exposed to 6 hours of heat (ambient air temperature 41°C, relative humidity 15%–25%), blood viscosity increased by 24%, red blood cell count increased by 9%, and platelet count rose by 18%. The authors speculated that in people with vascular disease, in whom vasodilation may be impaired and circulation reduced, such changes might explain the observed increase in thromboses. International experts agree that any factor compromising skin blood flow or normal cardiovascular function (such as a reduction in cardiac output) will augment the risk of heat-related injury and increase the need for careful monitoring. 37
Unfortunately, very few studies have examined the effects of heat exposure in this population. Those that have been performed have tended to include only participants with relatively mild cardiovascular disease and have therefore likely underestimated the magnitude of the effect in this vulnerable subgroup. Studies of passive heat exposure involving individuals with various cardiovascular impairments have found lower heart rates and lower systolic blood pressure than in healthy controls. 68,69 Cutaneous vasodilation and overall skin blood flow were also impaired, 70,71 but sweat rate was not affected. 69,70 In none of these studies were participants required to discontinue use of medications for their conditions (e.g., acetylsalicylic acid, angiotensin-converting-enzyme inhibitors, lipid-lowering medication), which might have confounded their responses to heat exposure. 47,48
Respiratory disease
Epidemiologic data indicate that patients with respiratory disease such as asthma, chronic obstructive pulmonary disease, lung cancer, influenza, pneumonia, bronchitis, tuberculosis and cystic fibrosis may be vulnerable to heat exposure 8,9 (Figure 1). A recent meta-analysis reported that individuals with pulmonary disorders are at a higher risk of death during heat waves (odds ratio 1.61, 95% confidence interval 1.2–2.1). 72 There is little evidence to elucidate whether this increase in mortality with heat exposure is due to the changes in air quality that often accompany warmer conditions 73 or to physiological failures in thermoregulation as a result of chronic conditions. In our literature search, we found no laboratory studies of thermoregulation in people with pulmonary disorders.
Socio-economic considerations
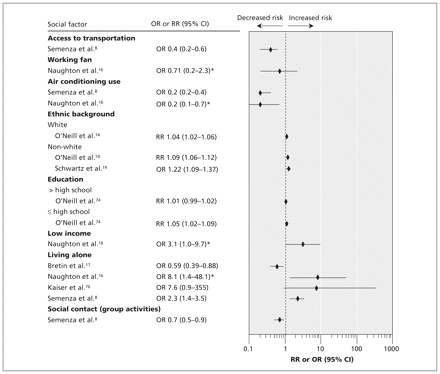
Ecological time-series and case–control studies of heat-related mortality have noted an unequal distribution of morbidity and mortality among various socio-economic groups. 2,4,19,74,75 A greater risk of mortality due to heat exposure has been associated with lower levels of education, 74 lower income, 16,75 being of nonwhite origin 4,19,74 and social isolation 8,16 (Figure 2). Working home air conditioners, 8,16 access to transportation 8 and access to cool environments during prolonged heat events (e.g., shopping mall, library) 8,16 have a substantial protective effect against heat-related deaths. Epidemiologic studies have suggested that air conditioning is associated with a relative risk reduction for heat-related illness of about 80%, and the presence of a working fan with a relative risk reduction of about 30%. 8,16
Figure 2: Relative risks (RRs) or odds ratios (ORs) for various social factors. RRs compare mortality during a heat wave with mortality during comparison dates without heat wave. ORs compare cases who died during a heat wave with controls in the same geographic area who did not die. Values greater than 1.0 indicate a greater risk of heat-related death. *Values were adjusted for neighbourhood and age. CI = confidence interval.
Housing quality may be a factor affecting the uneven distribution of morbidity and mortality during heat waves. 75 Those with lower income are more likely to live in crowded or poor-quality housing, where ventilation is inadequate and air conditioning nonexistent. 75 Homeless people can be especially prone to heat injury through lack of shelter from extreme heat or because of underlying chronic physical and psychiatric diseases. 4,8,9,72
Future research
Studies of thermoregulatory function in vulnerable populations have generally been limited to mild heat exposure or have involved low-risk patients. Very few studies have examined physical exertion in the heat involving people with obesity or chronic diseases. There is no information on whether combinations of various factors (e.g., older age and a chronic disease) compound the risk of heat-related morbidity and mortality. It will be essential to discern whether impairment in thermoregulatory capacity exists in terms of the whole-body response and not simply in terms of local heat-loss responses (sweating or skin blood flow or both). New research should focus not only on filling these gaps in the science-based information but also on developing clinical guidelines for health professionals, to facilitate the giving of advice to patients.
Conclusion
People with age over 60 years, obesity, hypertension, pulmonary or cardiovascular disease, or long-standing diabetes are at increased risk of heat-related illness — heat cramps, heat exhaustion and heatstroke — during prolonged heat events. Other contributing factors include homebound lifestyle, lack of contact with other people and decreased mobility. This increased vulnerability is related to physiologic al impairments in the regulation of body core temperature in hot conditions. Physicians should be aware of these risk factors and of protective factors against heat illness, such as fans, air conditioners and access to cool environments, and should counsel at-risk patients accordingly (Box 2).
Box 2: Suggestions for health care practitioners*
-
People with age over 60 years, obesity, cardiovascular disease, pulmonary disease or long-standing diabetes are at increased risk of heat-related illness during heat waves because of physiological impairments in the regulation of body core temperature in hot conditions.
-
A homebound lifestyle, lack of contact with other people and decreased mobility can also contribute to an increased risk of heat-related illness.
-
Working home air conditioners, fans, access to transportation and access to cool environments during prolonged heat events have a protective effect against heat-related illness and deaths.
-
Physicians should be aware of these risk factors and protective factors against heat illness, and should counsel at-risk patients accordingly.
Key points
Footnotes
-
This article has been peer reviewed.
Competing interests: None declared.
Contributors: Jane Yardley, Glen Kenny and Candice Brown were responsible for selecting the search terms, performing the database searches and assessing study quality. All of the authors were involved in the drafting and revision of the article and approved the final version submitted for publication.
Previously published at www.cmaj.ca
Acknowledgements: This research was supported by Health Canada, Climate Change and Health Office. Jane Yardley was supported by a Canadian Diabetes Association Doctoral Research Award and funds from the Ottawa Health Research Institute Research Chair in Lifestyle Research. Glen Kenny was supported by a University of Ottawa Research Chair Award. Ronald Sigal is supported by a Senior Health Scholar award from the Alberta Heritage Foundation for Medical Research
REFERENCES
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.