- © 2007 Canadian Medical Association
Abstract
Background: Human papillomavirus (HPV) is now known to be a necessary cause of cervical cancer, and prophylactic HPV vaccines aimed at preventing genital warts, precancerous cervical lesions and cervical cancer are now available. To gauge the potential impact on disease burden, we performed a systematic review of the evidence from randomized controlled trials.
Methods: We conducted a systematic search of the literature to identify all randomized controlled trials of prophylactic HPV vaccination. Reports in 5 electronic databases covering 1950 to June 2007 (MEDLINE, MEDLINE in process, EMBASE, the Cochrane Central Registry of Controlled Trials and the Cochrane Library), bibliographies of all included studies and of narrative reviews (2006–2007), clinical trial registries, Google Scholar, public health announcements, selected conference proceedings (2004–2007) and manufacturers' information on unpublished data or ongoing trials were screened against predefined eligibility criteria by 2 independent reviewers. Vaccines had to contain coverage against at least 1 oncogenic HPV strain. The primary outcome of interest was the frequency of high-grade cervical lesions (high-grade squamous intraepithelial lesion, or grade 2 or 3 cervical intraepithelial neoplasia). The secondary outcomes were persistent HPV infection, low-grade cervical lesions (low-grade squamous intraepithelial lesion or grade 1 cervical intraepithelial neoplasia), external genital lesions, adverse events and death. Meta-analysis of the data was done in all cases where adequate clinical and methodological homogeneity existed.
Results: Of 456 screened reports, 9 were included in the review (6 were reports of randomized controlled trials and 3 were follow-up reports of initial trials). Findings from the meta-analysis showed that prophylactic HPV vaccination was associated with a reduction in the frequency of high-grade cervical lesions caused by vaccine-type HPV strains compared with control groups: Peto odds ratio 0.14 (95% confidence interval [CI] 0.09–0.21) from combined per-protocol analyses, and 0.52 (95% CI 0.43–0.63) from modified intention-to-treat analyses. Vaccination was also highly efficacious in preventing other HPV-related infection and disease outcomes, including persistent HPV infection, low-grade lesions and genital warts. The majority of adverse events were minor. The incidence of serious adverse events and death were balanced between the vaccine and control groups.
Interpretation: Among women aged 15–25 years not previously infected with vaccine-type HPV strains, prophylactic HPV vaccination appears to be highly efficacious in preventing HPV infection and precancerous cervical disease. Long-term follow-up is needed to substantiate reductions in cervical cancer incidence and mortality.
Cervical cancer is an important cause of preventable cancer-related death among women. Because of the overwhelming burden of this disease in developing countries, cervical cancer is the second most common cause of cancer among women worldwide.1 It primarily affects women between 30 and 45 years of age, thereby representing in an important source of potential years of life lost.
With the obligate link between HPV and cervical cancer now established, prophylactic HPV vaccination represents a potential means of reducing the burden of cervical cancer and its precursor lesions. Two prophylactic HPV vaccines are now available. Both target HPV types 16 and 18, and one of the vaccines also targets HPV types 6 and 11. There are over 100 known subtypes of HPV, but types 16 and 18 are the most prevalent oncogenic strains of the virus, accounting for an estimated 70% of cervical cancers worldwide.1,2 Non-oncogenic strains, such as HPV types 6 and 11, are associated with the development of external genital disease, including genital warts. Most sexually active women will become infected with HPV in their lifetime, and over 50% of girls will acquire HPV within 48 months of becoming sexually active.3 Infection with an oncogenic strain of HPV does not guarantee that cervical cancer or HPV-related disease will develop. HPV-associated precancerous changes in the cervix either resolve spontaneously or may be identified through screening surveillance and treated.
Although 2 combined analyses of randomized trials of the quadrivalent vaccine were recently published,4,5 in this systematic review we summarize the body of existing evidence from randomized trials regarding the value of prophylactic vaccination against HPV as assessed by surrogate outcomes related to persistent HPV infection and precancerous lesions that lead to cervical cancer in women. We explicitly sought to determine whether women who receive prophylactic HPV vaccination have a lower incidence of persistent HPV infection and precancerous cervical lesions than women who are not vaccinated.
Methods
We conducted this systematic review based on a protocol developed a priori (the protocol is available from the corresponding author upon request). The QUOROM (Quality Reporting of Meta-analyses) statement was used to guide the content and reporting of the review.6
Search
The methods used for conducting and reporting the literature search followed the approach proposed by the STARLITE investigators.9 Using the OVID interface, we conducted electronic subset searches of 5 databases: MEDLINE (1950–2007 week 20), MEDLINE in process and other nonindexed citations (to June 20, 2007), EMBASE (1980–2007 week 21), the Cochrane Central Registry of Controlled Trials (to first quarter 2007) and the Cochrane Library. We also reviewed bibliographies of all included studies and of narrative reviews published in the last quarter of 2006 to May 2007, clinical trial registries, Google Scholar, public health announcements, selected conference proceedings (2004–2007) and information from vaccine manufacturers regarding any unpublished data or ongoing randomized controlled trials. For each electronic database, we developed an independent search strategy using relevant MeSH terms and terms identified from other reviews on HPV-related topics. Published search filters for identifying randomized trial evidence were applied to further refine the searches in both the MEDLINE and EMBASE databases.10,11 Subsets of records retrieved both with and without application of the search filters were examined to ensure that relevant records were not being missed. The electronic search strategy used for the MEDLINE database is in Appendix 1 (available online at www.cmaj.ca/cgi/content/full/177/5/469/DC2).
Selection
We considered eligible all published and unpublished reports of randomized controlled trials, with no exclusion on the basis of language or year of publication. Only studies involving women were included, with no exclusion on the basis of age or other demographic characteristics of the women enrolled. Interventions included any vaccine against HPV, as long as it contained activity against at least 1 oncogenic strain of the virus and was being administered with prophylactic intent. Reports of therapeutic vaccination were excluded. Any dosing regimen was considered acceptable. Comparators had to be either placebo or a “no HPV vaccination” group. Studies not designed to address outcomes related to vaccine efficacy against oncogenic HPV strains were excluded. The ultimate goal of HPV vaccination is to prevent death from cervical cancer. However, because cervical cancer is a rare event, even among women with persistent infection with an oncogenic HPV strain, and because following patients to a cancer outcome is not ethically justifiable, clinically relevant surrogate outcomes were evaluated. Cervical cancer develops in a progressive fashion and, as such, we selected clinical outcomes along the following continuum of the natural history of HPV infection: incident HPV infection, persistent HPV infection, low-grade cervical lesions (low-grade squamous intraepithelial lesion or grade 1 cervical intraepithelial neoplasia) and high-grade cervical lesions (high-grade squamous intraepithelial lesion, or grade 2 or 3 cervical intraepithelial neoplasia). High-grade cervical lesions were chosen as the primary outcome of interest because they are the immediate precursors to cervical cancer. Furthermore, the US Food and Drug Administration and the World Health Organization have previously recommended the use of grade 2 or 3 cervical intraepithelial neoplasia as a surrogate outcome for cervical cancer in HPV vaccine trials.7,8 The secondary outcomes we evaluated included persistent HPV infection, low-grade cervical lesions, external genital lesions, serious adverse events and death. We excluded immunogenicity and dose-finding studies.
Validity assessment
The collective set of identified reports was assembled in Reference Manager (version 10; Thomson ISI ResearchSoft, Carlsbad, Calif.) and duplicate references were removed manually. All levels of screening were performed in duplicate by 2 of us (L.R. and L.H.) as the primary reviewers. Excluded records were assembled in separate files with reasons for exclusion documented in all cases. Records agreed upon for exclusion by both reviewers were eliminated at this stage. Full-text articles were obtained for all records not eliminated at this step, including any discrepancies. The same 2 reviewers subsequently assessed the full-text articles according to the inclusion and exclusion criteria for the review. Discrepancies were resolved by means of consensus, and by a third-party reviewer if necessary.
The 2 primary reviewers performed quality assessments independently using the scale previously validated by Jadad and colleagues.12 Separate assessments were made for the reporting of allocation concealment, because inadequate reporting may result in an overestimation of intervention effects.6 With respect to outcome-level assessments, various factors with the potential to introduce bias into the estimates of effect were considered and described.13 Any discrepancies in quality scoring or assessments were resolved by means of consulting a third-party reviewer. The quality-assessment process was not blinded because there is no convincing evidence that such blinding affects the results of meta-analyses.12,14
Data abstraction
Data abstraction was performed in duplicate using a data abstraction form developed and piloted by both primary reviewers. Data collected from each report included demographic characteristics pertaining to the women in the study (e.g., age, ethnic background, baseline HPV status, prior cervical abnormalities, number of sexual partners), details regarding the vaccine preparation used (e.g., HPV strain coverage, dose, schedule of administration, comparator) and the outcomes assessed (e.g., primary versus secondary outcomes, outcome definitions, assessment timelines). Data regarding duration of follow-up, study results and conclusions, funding sources and study quality were also recorded.
Data synthesis
Qualitative synthesis of the abstracted data was completed for all included studies with respect to study populations, interventions and outcomes. Where studies were sufficiently homogeneous to support quantitative synthesis (populations, interventions, outcomes and study methods), we performed a meta-analysis and constructed forest plots. Sources of both clinical and methodological heterogeneity were considered in the review and described. Because the quantitative outcomes of interest deal with rare events, effect sizes are presented as Peto odds ratios with associated 95% confidence intervals.15 A Peto odds ratio of less than 1.0 suggests that fewer events were seen among vaccinated women than among those in the control group, whereas a Peto odds ratio of 1.0 or more suggests the reverse. Pooled estimates and their confidence intervals were obtained using a fixed-effects model. We evaluated statistical heterogeneity by examining the overlap of confidence intervals between individual studies. In addition, we quantified the degree of variability in effect estimates attributable to heterogeneity using the I2 statistic.
Results
Trial flow
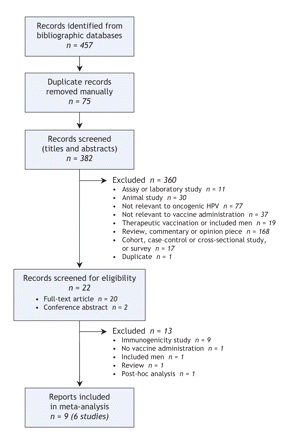
A flow diagram detailing the process for the selection of records relevant to our review is outlined in Figure 1. Of the 457 records initially identified through the full search strategy, 448 were subsequently excluded. The most common reasons for exclusion were that the reports were review articles or commentaries, the studies were not related to oncogenic HPV or vaccine administration, the studies involved animals, or they were laboratory studies or immunogenicity investigations. Of the 9 reports included,16–24 3 were of follow-up data from prior studies.19,21,24 A total of 6 studies are therefore represented by the 9 reports. All 6 studies included in the systematic review were randomized controlled trials that involved the administration of a vaccine to women for the purpose of prophylaxis against oncogenic HPV-related infection and disease.
Figure 1: Selection of studies for meta-analysis.
Study characteristics
A total of 40 323 participants were enrolled in the 6 studies. The ages of the women ranged from 15 to 25 years. Their ethnic background was primarily white, but there were also women of Hispanic, Asian and black descent. Recruitment was multinational and took place primarily in North America, Latin America, Asia Pacific and Europe. The earliest study began recruiting women in the latter part of 1998. The greatest number of lifetime sexual partners of the participants was 6, and the majority of the women (> 90%) had had no prior abnormal Papanicolaou (Pap) test results. Participants without prior sexual activity were enrolled if they were seeking contraception. All of the vaccines administered contained coverage against HPV type 16. In general, women were assessed at 6-month intervals. Further details of the characteristics of the included studies are presented in Table 1.
Table 1.
All 6 studies were of high methodologic quality (score of 5/5 on the Jadad scale12). Adequate allocation concealment was reported in most instances. The studies reported results according to per-protocol and modified intention-to-treat assessments, and the per-protocol assessments were most commonly used for the primary analysis. Per-protocol assessments evaluated the prophylactic efficacy of vaccination in women uninfected at baseline with an HPV strain covered by the vaccine and who received all 3 doses of the vaccine. Outcome events were counted starting at month 7. Modified intention-to-treat assessments included women who had received at least 1 vaccine dose but who may have become infected before receiving all 3 doses. Outcome events were counted starting 1 month after the first dose of vaccine (Table 2). Outcome-level assessment involved examination of the numbers of participants included in the analyses compared with the number in the initial randomization. Among the published follow-up reports,19,21,24 between 40% and 70% of the women in the initial randomization completed the extended follow-up phases of the study. Per-protocol analyses included 63%–87% of the participants who were in the initial randomization. Modified intention-to-treat analyses included 84%–100% of those who were in the initial randomization. Not all of the participants included in the analyses had been followed for the full follow-up period (Table 3).
Table 2.
Table 3.
Data synthesis
High-grade cervical lesions
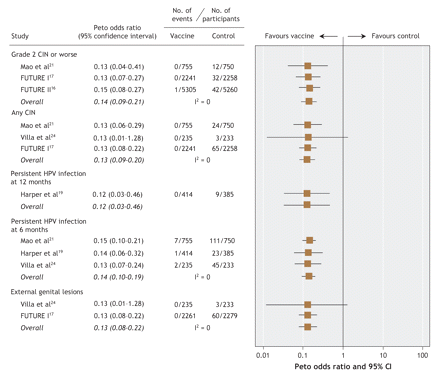
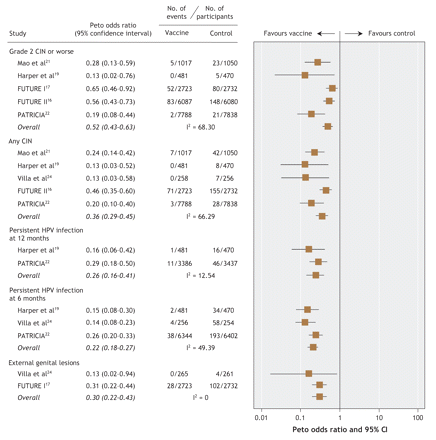
Five of the 6 included studies reported outcomes for high-grade cervical lesions.16,17,19,21,22 Two studies — the FUTURE (Females United to Unilaterally Reduce Endo/Ectocervical Disease) II trial16 and the PATRICIA (PApilloma TRial against Cancer In young Adults) study22 — evaluated high-grade lesions as their primary efficacy objective. The longest mean duration of follow-up among the published reports was 47.7 months. A recent abstract of additional follow-up from one of the studies19 reported continued vaccine efficacy up to 5.5 years.25 It was not possible to pool the abstract results in the meta-analysis because population sizes were not reported. Among studies pooled in the per-protocol meta-analysis, 1 case of high-grade lesion was reported among the vaccinated women, as compared with 86 among the controls. The pooled, overall Peto odds ratio was 0.14 (95% confidence interval [CI] 0.09–0.21) in the per-protocol analysis (Figure 2) and 0.52 (95% CI 0.43–0.63) in the modified intention-to-treat meta-analysis (Figure 3).
Figure 2: Per-protocol meta-analysis of clinically important outcomes in selected studies of prophylactic vaccination against human papillomavirus (HPV)-related infection and disease. (Per-protocol analyses included study participants who were seronegative and DNA negative for relevent HPV types at enrolment and who received all 3 doses of vaccine.) CIN = cervical intraepithelial neoplasia.
Figure 3: Modified intention-to-treat meta-analysis of clinically important outcomes in selected studies of prophylactic vaccination against human papillomavirus (HPV)-related infection and disease. (Modified intention-to-treat analyses included study participants who received at least 1 dose of vaccine and who either were negative for relevant HPV types at enrolment [Harper, Villa, PATRICIA] or were randomly assigned to study group irrespective of their baseline HPV status [Mao, FUTURE I, FUTURE II].) CIN = cervical intraepithelial neoplasia.
Any cervical lesions
Five of the 6 studies reported outcomes pertaining to any grade of cervical lesion or worse.17,19,21,22,24 This comprised low-grade lesions, high-grade lesions, carcinoma in situ and cancerous lesions. Pooled estimates of efficacy were associated with an overall Peto odds ratio of 0.13 (95% CI 0.09–0.20) in the per-protocol analysis (Figure 2) and 0.36 (95% CI 0.29–0.45) in the modified intention-to-treat analysis (Figure 3).
Persistent HPV infection
Persistent infection with HPV types 16 and 18 was reported both as a 6-month outcome (Harper and associates,18,19 Koutsky and colleagues20/Mao and colleagues,21 PATRICIA study22 and Villa and associates23,24) and as a 12-month outcome (Harper and associates18,19 and PATRICIA study22). Pooled estimates of vaccine efficacy using a 6-month criterion for the definition of persistence resulted in an overall Peto odds ratio of 0.14 (95% CI 0.11–0.19) in the per-protocol analysis and 0.22 (95% CI 0.18–0.27) in the modified intention-to-treat analysis. With respect to the 12-month criterion for persistent infection, the pooled estimates of vaccine efficacy were similar. Harper and associates19 reported no persistent infections with HPV type 16 or 18 among vaccine recipients, as compared with 9 cases in the control group (per-protocol analysis). In the modified intention-to-treat analysis, there were 12 cases of infection persistent for 12 months among the vaccine recipients versus 62 cases among the controls.19,22 The overall Peto odds ratios were 0.12 (95% CI 0.03–0.46) and 0.26 (95% CI 0.16–0.41) in the per-protocol and modified intention-to-treat analyses respectively (Figure 2 and Figure 3).
External genital disease
Two of the studies reported outcomes for external genital disease (genital warts, vulvar intraepithelial neoplasia or vaginal intraepithelial neoplasia).17,24 Villa and associates24 reported no cases of genital warts among vaccine recipients in both the per-protocol and modified intention-to-treat analyses. In the FUTURE I study,17 no cases of external genital lesions were reported among the vaccine recipients in the per-protocol analysis, as compared with 60 cases among the controls; the corresponding numbers in the modified intention-to-treat analysis were 28 and 102 cases of external genital lesions. In the combined analysis, the overall Peto odds ratios were 0.13 (95% CI 0.08–0.22) and 0.30 (95% CI 0.22–0.43) in the per-protocol and modified intention-to-treat analyses respectively (Figure 2 and Figure 3).
Adverse events and death
In the majority of studies, participants were asked to keep a standard diary card for 7–14 days following vaccination. Adverse events were generally mild, and the most commonly reported adverse events were reactions at the injection site. Headache, fatigue and myalgia were the most commonly reported systemic adverse events. Gastrointestinal complaints and itching were also frequently reported, and temperature elevations were reported in about 15% of women.17,18 Serious adverse events were reported with similar frequency in the vaccine and control groups, and those considered possibly related to the vaccine included bronchospasm, gastroenteritis, headache, hypertension, and pain at the injection site or impaired joint movement in the injected limb. The follow-up study by Harper and associates,19 based on a mean follow-up of 47.7 months, also reported cases of new-onset chronic disease reported over the full duration of follow-up. Ten (3%) of the women in the vaccine group and 18 (5%) in the control group reported the onset of a new chronic disease. The meta-analysis demonstrated that, overall, the incidence of serious adverse events and death was balanced between the vaccine and control groups (Peto odds ratio for serious adverse events 0.998, 95% CI 0.87–1.14; for death 0.91, 95% CI 0.39–2.14) (Figure 4). Most deaths were reported as accidental, and none of the deaths was considered attributable to the vaccine.
Figure 4: Meta-analysis of serious adverse events and death in selected studies of prophylactic vaccination against HPV-related infection and disease.
Interpretation
Our systematic review demonstrates that prophylactic HPV vaccination is highly efficacious in preventing vaccine type-specific HPV infection and precancerous cervical disease, particularly among women aged 15–25 years who received all 3 vaccine doses, had no more than 6 lifetime sexual partners and had no prior abnormal results from Pap screening. From these studies, the per-protocol analyses permit estimation of the effect of vaccinating young girls before becoming sexual active (and thus before HPV exposure) through the use of programs that are able to ensure compliance with the full vaccination schedule. The modified intention-to-treat analyses provide effect estimates that relate to more heterogeneous and potentially less compliant populations.
Clinical outcomes that occur early in the natural progression of HPV-related disease, such as incident and persistent HPV infections, were evaluated primarily in the initial phase II studies.18–21,23,24 Among the participants who had no HPV infection at the time of vaccination, vaccination was consistently efficacious across the studies in preventing persistent HPV infection. Although persistent HPV infection may be associated with progression to precancerous and cancerous lesions, spontaneous resolution is common and may take more than a year. A criterion of 4–6 months for the definition of persistent infection was therefore unlikely to permit sufficient time to distinguish between regressing and progressing lesions. Demonstration of vaccine efficacy versus these early HPV outcomes was primarily valuable in justifying subsequent studies that evaluated more clinically meaningful outcomes.
For the outome “any cervical lesion or worse,” vaccination appeared to be highly efficacious among women who received all 3 doses of vaccine. The combined evaluation of all detectable precancerous cervical lesions may provide useful information to gauge resource and cost implications associated with proposed vaccine implementation. Collectively, “any cervical intraepithelial neoplasia” corresponds with the full range of lesions detectable through current cervical screening practices in much of the developed world. In these environments, fewer cervical lesions detected among women may result in substantially reduced costs and resource use. In the developing world, where organized screening remains largely unavailable, women are likely to derive benefit only if fewer cases of cervical intraepithelial neoplasia, achieved through vaccination, translates into fewer cases of invasive cervical cancer.
Cervical lesions that are histologically high grade in nature are associated with significant malignant potential. These lesions are typically detected through Pap screening, and definitive treatment is recommended and standard. Consequently, high-grade disease represents the most clinically meaningful surrogate outcome for cervical cancer in prophylactic HPV vaccination trials. Therefore, we chose it as the primary outcome of interest for our systematic review. The large phase III trials — FUTURE II16 and PATRICIA22 — both evaluated a primary outcome of high-grade precancerous disease. Both trials demonstrated high efficacy against cervical lesions of grade 2 or worse related to HPV types 16 and 18 based on a mean follow-up of 3 years (FUTURE II) and 15 months (PATRICIA). The longest mean follow-up for any trial to date is 5.5 years.25 Additional follow-up from clinical trials remains critical to substantiate long-term vaccine efficacy. Furthermore, the intense follow-up schedule for assessments in these studies, which exceeds that of most practice settings, would tend to pick up earlier outcome events in the natural progression of HPV-related infection and disease and would consequently result in an underestimation of more clinically meaningful disease outcomes. This serves to highlight the importance of the next phase of evaluating vaccine efficacy in large studies designed to closely reflect real-world settings.
Considerable resources are spent each year on the management of external genital disease.26 Among the studies included in our review, substantial reductions in the incidence of external genital lesions were demonstrated following administration of vaccines with coverage against HPV types 6 and 11. Conclusions regarding individual types of lesions (genital warts, and vulvar or vaginal intraepithelial neoplasia) are somewhat limited by the use of a composite of these clinical outcomes among the studies. Country-specific data regarding the burden of disease and resource use associated with the management of external genital disease will be helpful to assess the impact of vaccine coverage for these HPV strains.
Interpretation of the results of our systematic review requires consideration of several limitations. We did not find evidence that prophylactic vaccination against HPV types 16 and 18 reduces cervical cancer incidence or mortality. Inferences regarding these outcomes rely on the strength of the surrogate outcomes related to these oncogenic strains that were evaluated in the studies. In addition, the external validity of the results relates to the women included in the studies, who were 15–25 years of age, were mostly white, were mostly from developed nations and were mainly otherwise healthy. Indeed, women were recruited primarily from colleges and universities. Further research is needed to demonstrate efficacy in more representative populations of women and men. The possible implications of vaccinating against only 2 oncogenic HPV strains must also be considered, as must the duration of vaccine efficacy. Limitations regarding the methodologic quality of the studies reviewed relate primarily to participants lost over time or excluded from the analyses, or both. Less than half of the participants included in the initial randomization completed the full follow-up in some cases, and up to 35% were excluded from some of the analyses. It is difficult to preserve the benefits of randomization given the magnitude of participants unaccounted for. Another limitation is that we did not evaluate outcomes specific to HPV subtypes. In addition, bias may have been introduced in the modified intention-to-treat analysis given the heterogeneity among studies with respect to baseline HPV status of the participants at enrolment.
In summary, our systematic review demonstrates that prophylactic HPV vaccination is highly efficacious in preventing vaccine type-specific HPV infection and precancerous cervical disease, particularly among women aged 15–25 years with no prior abnormal results from Pap screening and no more than 6 lifetime sexual partners. Conclusions regarding the prevention of early events in the natural progression of HPV-related disease are robust. Vaccination appears to be well tolerated and safe. Data to help reconcile the gap between the impressive vaccine efficacy demonstrated in these trials and the potential effectiveness of vaccination at reducing the global burden of cervical cancer and death from the disease should be forthcoming from phase IV trials currently underway.
Footnotes
-
Une version française de ce résumé est disponible à l'adresse www.cmaj.ca/cgi/content/full/177/5/469/DC1
This article has been peer reviewed.
Contributors: Lisa Rambout was responsible for the conception and design of the review and contributed to the literature search and screening of reports, the abstraction, analysis and interpretation of the data, and the drafting and revision of the manuscript. Laura Hopkins contributed to the screening of reports, the abstraction and interpretation of the data, and the critical review and revision of the manuscript. Brian Hutton contributed to the analysis of the data and the revision of the manuscript. Dean Fergusson contributed to the design of the review, the interpretation of the data and the critical review and revision of the manuscript for methodological content and accuracy. All of the authors gave final approval of the version to be published.
Acknowledgements: We thank Risa Shorr, Library Services, The Ottawa Hospital, for her assistance with the search strategy and acquisition of articles. We also thank David Moher for education in the conduct of systematic reviews during course work in the University of Ottawa's Master's in Epidemiology and Community Medicine program.
Competing interests: None declared for Lisa Rambout, Brian Hutton or Dean Fergusson. Laura Hopkins received speaker fees from Merck Frosst Canada Ltd. for educational sessions held in November 2006 and May 2007 for clinicians on HPV epidemiology and vaccine development.