- © 2007 Canadian Medical Association or its licensors
Prognosis after cardiac arrest is dismal, with an overall survival rate of less than 6%. Outcomes vary with cardiac arrest rhythm: less than 2% of patients with asystolic or pulseless electrical activity survive, compared with up to 33% of patients with ventricular fibrillation or pulseless ventricular tachycardia. Advanced cardiac life-support algorithms focus on early aggressive resuscitation; unfortunately, most patients who survive do sustain anoxic brain injury. About 60% of cardiac arrest survivors regain consciousness; of these, one-third experience irreversible cognitive disabilities.
Treatment options for anoxic brain injury traditionally have been limited to supportive care. Recently, however, induced hypothermia has been shown to reduce the effects of anoxic brain injury and has become a management option when patients are resuscitated after cardiac arrest. Although the mechanism is incompletely understood, a reduction in core body temperatures is thought to diminish cell injury and increase cerebral neuronal healing by reducing cerebral oxygen demand and intracranial pressure, which may improve postischemic hypoperfusion, stabilize plasma membranes and suppress the production and release of free radicals.
In this article, we briefly review evidence that supports a position statement recently released by the Canadian Association of Emergency Physicians on induced hypothermia of adults with cardiac arrest.1 We then provide practical advice for its application.
The supporting evidence
The clinical application of hypothermic modulation of anoxic brain injury was initiated in the 1950s. Its current use is supported by 2 prospective randomized controlled trials: the Hypothermia after Cardiac Arrest (HACA) trial2 and the Bernard trial3 (Table 1). These studies enrolled comatose patients resuscitated from ventricular fibrillation or pulseless ventricular tachycardia. All patients received advanced cardiac life-support resuscitation and postresuscitation care. All were sedated, paralyzed and ventilated. Those in the experimental group also underwent induced hypothermia to 32°C–34°C within 6 hours of arrest. Although the trials were similar, their hypothermia protocols differed somewhat (Table 1).1,2
Table 1.
Mild hypothermia in both trials was associated with fewer deaths and reduced disability. As summarized in Table 1, the number-needed-to-treat (i.e., to prevent 1 death) in the HACA trial was 7; the smaller Bernard trial showed a nonsignificant trend in favour of the hypothermia group. Reductions in disability were statistically significant in both trials: the number-needed-to-treat to reduce neurologic impairment in the HACA trial was 6; to discharge 1 additional patient home or to a rehabilitation facility in the Bernard trial, 4. Although the differences in adverse events between the hypo-and normothermia groups in either trial were nonsignificant, the HACA study noted a trend toward increased sepsis in the hypothermia group.
Although well designed, these trials were limited by their use of stringent enrolment criteria (Fig. 1). In the HACA trial, only 275 of the 3551 patients assessed for eligibility were enrolled; this proportion was not described for the Bernard trial. As well, the lack of blinding in both trials introduces a potential for observer bias.
Fig. 1: The Canadian Association of Emergency Physicians algorithm for the institution of induced hypothermia. Adapted, with permission, from the Canadian Journal of Emergency Medicine (CJEM 2005;7:42-7).
*Best evidence of benefit is in survivors of cardiac arrest secondary to ventricular fibrillation or tachycardia. There is theoretical benefit to survivors of other primary cardiac arrest (i.e., non–ventricular fibrillation/tachycardia rhythms) if they meet other eligibility criteria. The decision to initiate hypothermia in these patients should be made in consultation with receiving staff in the emergency department or intensive care unit.
Because of findings from these important trials, the International Liaison Committee on Resuscitation has recommended hypothermic modulation of anoxic brain injury as the standard of care (level 1 evidence) for resuscitated survivors of out-of-hospital cardiac arrest with ventricular fibrillation or tachycardia.4 On the basis of available data, the Canadian Association of Emergency Physicians has also issued a position statement that supports the use of hypothermic modulation of anoxic brain injury.1,5
The induction procedure for mild hyperthermia
As summarized in Fig. 1, adult patients who are resuscitated from cardiac arrest with primary ventricular fibrillation or pulseless ventricular tachycardia and are comatose are optimal candidates for induced hypothermia. Patients with a Glasgow Coma Scale score of less than 10 without rapid improvement in their level of consciousness have evidence of anoxic brain injury and should be considered for induced hypothermia. Other cardiac arrest rhythms, such as asystole or pulseless electrical activity, have not been sufficiently studied to recommend the routine use of hypothermic modulation of anoxic brain injury. However, the pathophysiology of anoxic brain injury is similar to that in survivors of ventricular fibrillation or tachycardia; cooling may therefore be useful in patients who require supportive care.
Patients whose resuscitation efforts are prolonged (> 60 min from collapse to return of spontaneous circulation) or who have ongoing hemodynamic (systolic blood pressure < 90 mm Hg) or respiratory insufficiency (O2 saturations < 85% for > 15 min) are poor candidates for induced hypothermia (Fig. 1, exclusion criteria). Cardiac arrests not due to a primary myocardial event (e.g., arrests caused by toxic ingestion, trauma or submersion) have been insufficiently investigated.
To minimize ongoing neuronal damage after the return of spontaneous circulation, cooling should be instituted as soon as possible. Every effort should be made to reduce the patient's core temperature to 32°C–34°C within 6 hours of the event.
The most practical approach to cool patients is with ice packs applied to the patient's head, neck, axillae and groin, as described in the trial reports. Plastic bags should be partially filled with ice, and water added to increase contact with the patient. A barrier such as a cotton or flannel sheet placed between ice packs and the patient's skin will minimize the risk of frostbite injury.
The number of ice packs required varies from 20 to 50, depending on the size of the patient. If more ice is needed, most hospital cafeterias can provide it. Alternative cooling methods include the infusion of a cooled intravenous fluid (e.g., 2–3 L of normal saline at 4°C) or use of commercially available intravascular devices, cooling blankets, fans and mist.
Core body temperature should be maintained in the range of 32°C–34°C for 12–24 hours. Patients require a monitored setting with expert nursing and physician care. Continuous cardiac and oxygenation saturation monitoring is recommended, with continuous or half-hourly measurements of core temperature to ensure optimal regulation. Temperature measurements must be accurate; they can be obtained by rectal, esophageal, oral or more invasive means (e.g., by bladder or pulmonary artery catheter). Infrared assessment of the temperature of the tympanic membrane does not accurately reflect the core temperature of the body, and should not be used.
Core temperatures below 32°C are associated with increased adverse events such as dysrhythmias, reduced cardiac function, hypotension, immune suppression and coagulopathy. If a patient's core temperature falls below 32°C, ice packs or other cooling modalities should be discontinued temporarily until core temperatures return to the 32°C–34°C range.
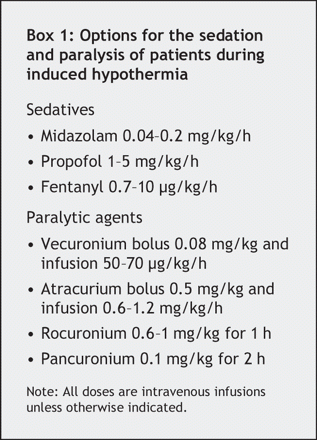
Eligible patients are generally intubated. To inhibit shivering and to ensure patient comfort, comatose survivors of cardiac arrest require adequate sedation and paralysis (Box 1). Shivering is common; not only is it uncomfortable for the patient, it impedes efficient cooling.
Adverse events
Possible adverse events include not only local effects of the application of ice but also systemic effects on various physiologic systems such as the cardiovascular, hematological and immune systems. However, there is little evidence that clinically significant adverse events occur with mild hypothermia; in the HACA trial, for example, adverse events occurred in 73% of the hypothermic and 70% of the normothermic group (p = 0.70).2 The most important adverse events included hypothermia-associated sepsis (13% of the hypothermic v. 7% of the normothermic group), coagulopathy (26% v. 19%) and cardiac dysrhythmias (36% v. 32%).2
To ensure that adverse events are readily identified, patients who undergo induced hypothermia should have daily measurements of their complete blood count, international normalized ratio, partial thromboplastin time and serum electrolyte concentrations. Clinicians caring for these patients must monitor for adverse events and abandon active cooling if the risks outweigh the benefits.
On occasion, survivors of cardiac arrest may require interventions such as thrombolysis or percutaneous coronary angiography and stenting. Whether the efficacy of these interventions is affected by induced hypothermia is not known. Although data on the use of induced hypothermia is incomplete in these cases, optimal cerebral outcomes must be considered, and induced hypothermia should not be delayed for the institution of other interventions.
Conclusion
Induced hypothermia should be considered for cardiac arrest survivors, and cooling instituted expediently.
Footnotes
-
This article has been peer reviewed.
Competing interests: None declared.