Abstract
Primary care physicians see many of the estimated 250 000 Canadians chronically infected with the hepatitis C virus (HCV). Of this number, about one-third are unaware they are infected, which constitutes a large hidden epidemic. They continue to spread HCV unknowingly and cannot benefit from advances in antiviral therapy that may clear them of the virus. Many HCV-infected people remain asymptomatic, which means it is important to assess for risk factors and test patients accordingly. The third-generation enzyme immunoassay for HCV antibodies is a sensitive and specific test, although the presence of the virus can be confirmed by polymerase chain reaction testing for HCV RNA in some circumstances. Pegylated interferon-α and ribavirin combination therapy clears the virus in about 45%–80% of patients, depending on viral genotype. Preventive strategies and counselling recommendations are also reviewed.
Hepatitis C virus (HCV), which was first characterized in the late 1980s, is an RNA flavivirus with 6 major genotypes and more than 50 subtypes.1 Genotype 1 is predominant in Canada, accounting for over 60% of cases, followed by type 2 (11%–16%), type 3 (6%–14%), and the uncommon types 4, 5 and 6 (< 5%).2,3,4 It is estimated that 123–170 million people5,6 worldwide are living with HCV infection, of whom 250 000 (0.8%) are in Canada,7 and that about 5000 Canadians are newly infected each year.7 There have been major antiviral treatment advances in recent years. No vaccine is available, although vaccine research is underway. The goal to reduce the number of people living with hepatitis C and its complications cannot be achieved without the contributions of public health, specialty care and primary care clinicians. The primary care approach to the prevention, screening, diagnosis and management of HCV infection among adult patients is the focus of this review.
Epidemiologic features
HCV is primarily transmitted parenterally. In Canada, recreational injection drug use (IDU) has been and continues to be the dominant mode of HCV acquisition. Data from the 8-centre Laboratory Centre for Disease Control Sentinel Surveillance from 1993 to 1995 were analyzed for a detailed risk factor profile of 585 HCV-infected patients.8 Of these patients, 67% had a history of IDU, 17% of both IDU and blood transfusion and 6% of transfusion but no IDU. Therefore, 84% of HCV-infected patients involved this study admitted to IDU. In a sample of 698 patients in Vancouver between 1995 and 1996, 70% had a history of IDU and 16% had received a blood transfusion. The proportion of people with both risk factors was not reported.8 In the largest published Canadian series of 239 patients from a Calgary hepatology practice between 1995 and 1999, 74% had a history of IDU or cocaine inhalations.9 IDU was also the predominant risk factor in 2 smaller series, from an Ottawa gastroenterology practice10 and the Prince Edward Island Public Health Unit.11 It thus appears that IDU is the route of transmission for about 70%–80% of the recent HCV cases in Canada. Practitioners must ask about any history of IDU, even a single episode in the remote past, by patients confirmed or suspected to be infected with HCV. Many patients are surprised to discover that they are chronically infected with a virus that was transmitted several decades earlier, during an experience that happened only once and that seemed to be innocuous or trivial.
In the larger cities of Canada, the second largest risk factor is travel or residence in a region in which HCV infection is endemic (Table 1). In many countries, especially in the past, parenteral injections were done with reusable glass syringes and needles. Compelling epidemiologic data indicate that such health care-related parenteral injections account for the high HCV seroprevalence rates in areas such as Egypt, Pakistan, Asia, and, in the past, southern Italy and Japan.8,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32 Sadly, such transmission continues today in many resource-poor countries; the World Health Organization estimates that globally 8–12 billion injections are given every year, with more than half of these being unsafe in the developing world; 2–5 million HCV infections annually are attributable to unsafe health care injections.18,19,20,28,33 Some countries have massive HCV problems: about 10% of the Pakistani population is infected, as is about 15%–20% of the population in Egypt.12,16 Most patients in Egypt became infected during attempts to eradicate endemic bladder schistosomiasis by mass parenteral antischistosomal treatment programs.16,17
Table 1.
Transmission through blood products and organ transplantation has been virtually eliminated in North America since 1990, following the exclusion of high-risk donors, new viral inactivation procedures, and the development of increasingly more sensitive HCV screening tests.34,35,36 The current risk of HCV infection from blood transfusion in Canada is estimated to be less than 1 in 3 000 000 units transfused.37
Sexual and vertical transmission of HCV is uncommon and much less efficient than with hepatitis B virus or HIV.32,38,39 High HCV prevalence is found among sex workers, but it is often linked to drug injection and to people who have multiple partners.40,41,42 In Western countries, the prevalence among long-term sexual partners with continuing sexual exposure and no other risk factors is relatively low (< 5%).43,44,45 Recently, apparent sexual transmission of HCV has been increasingly reported in Europe among gay men who are not injection drug users; such men have a higher prevalence of fisting and of lymphogranuloma venereum, HIV and other sexually transmitted infections.46,47,48,49 The vertical HCV transmission risk is estimated to be about 4%–7%, and it rises with HIV coinfection and a high maternal HCV viral load.50,51,52 HCV is not transmitted by breastfeeding.52,53
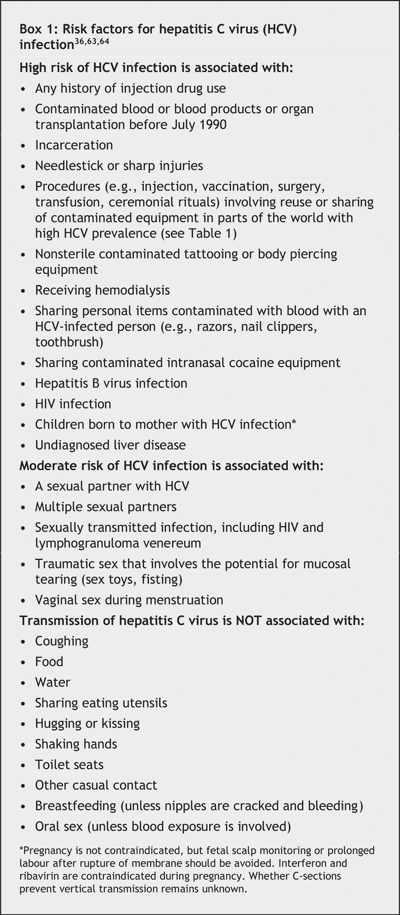
Risk of HCV transmission through needlestick injury is in the order of 1%–3%, compared with 30% for hepatitis B virus and 0.3% for HIV.54,55,56 Transmission has also been associated with organ transplantation, hemodialysis, tattooing, and sharing contaminated equipment in parts of the world where the HCV prevalence is high.12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,57,58,59,60 Sharing contaminated intranasal cocaine equipment (straw or rolled-up money) may be associated with transmission.61,62,63 Coughing, hugging, kissing, shaking hands, sharing eating utensils or casual contact does not transmit HCV, nor is it transmitted through food or water (Box 1). The mean incubation period is 6–8 weeks.65,66,67
Natural history and clinical manifestations
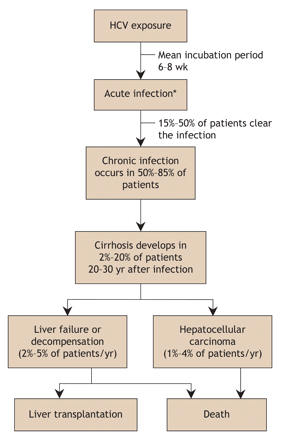
The natural history of HCV infection is shown in Fig. 1. Most infected people (60%–75%) do not experience symptoms when acutely infected.69 For those with symptomatic acute infections, the manifestations are similar to those of hepatitis A or B virus infection: malaise, fatigue, lethargy, anorexia, abdominal pain, jaundice, mild hepatosplenomegaly, maculopapular rash and arthralgia. These symptoms may last for 2–12 weeks. Patients with acute viral hepatitis often report a transient distaste for cigarettes or alcohol or both. Fulminant hepatitis is very rare in the acute infection stage.63,68,70 It must be emphasized that physicians, unless they work at an inner-city clinic with a high prevalence of active injection drug users, are unlikely to come across patients with symptoms of acute hepatitis. This is because the vast majority of acute HCV cases are asymptomatic or have only mild flu-like symptoms with little or no jaundice.
Fig. 1: Natural history of hepatitis C virus (HCV) infection.68 *Note that 60%–75% of patients are asymptomatic at this stage.
The differential diagnosis of a symptomatic acute HCV infection includes alcohol and other substance use, medication or supplement use, hepatitis A, B, D or E, primary biliary cirrhosis, autoimmune hepatitis, fatty liver, hemochromatosis, Wilson's disease and α-1 antitrypsin deficiency.
A minority of newly infected patients (15%–50%) will clear the infection, but in most (50%–85%) the infection will become chronic. The risk of chronicity after acute infection is less (50%–70%) with community-acquired infections and higher (70%–85%) in post-transfusion cases. Some patients with chronic infection experience malaise, nausea, abdominal pain and pruritis. Fluctuating alanine transaminase levels are characteristic. The physical examination may reveal signs of liver disease such as spider angiomata, palmar erythema and telangiectasia. Much later in the course, if advanced cirrhosis develops, jaundice, splenomegaly, ascites, esophageal varices and hepatic encephalopathy may be noted. Extrahepatic manifestations are uncommon and may include mixed essential cryoglobulinemia, membranous or membranoproliferative glomerulonephritis, non-Hodgkin's lymphoma, Sjögren's syndrome, lichen planus and porphyria cutanea tarda.71,72,73,74,75,76,77,78,79,80
Chronic infection causes mild chronic inflammation of the liver. Ongoing cycles of inflammation, necrosis and apoptosis eventually lead to scarring (fibrosis) and, ultimately, severe bridging fibrosis with nodular regeneration. This, of course, is cirrhosis. The rate at which fibrosis progresses to cirrhosis in chronic HCV infection is relatively slow compared with that of many other liver diseases. Factors that increase the progression rate include sex (men > women), older age at infection acquisition, longer duration of infection, immune suppression (e.g., HIV–HCV coinfection), chronic hepatitis B coinfection, moderate or heavy alcohol use, and obesity.68,81,82,83,84,85,86 Over 20–30 years of infection, the risk of cirrhosis is 2%–20%; after cirrhosis has developed, the risk of hepatocellular carcinoma is 1%–4% each year.68,87 Hepatitis C infection is currently the leading indication for liver transplantation in North America,63,88,89 not because of rapid progression to cirrhosis and end-stage liver failure but mostly because of the large number of people with chronic infection (3 million in North America), many of whom have been infected for 3 or 4 decades.
Serologic testing
Although there are no data evaluating the efficacy of screening and treatment on the basis of long-term outcomes (e.g., liver transplant demand, survival),90,91 the Canadian Consensus Conference on Viral Hepatitis Management and the US Centers for Disease Control and Prevention and National Institutes of Health recommend the serologic testing of people at increased risk of hepatitis C infection (Box 1).63,70,92,93 This recommendation is based on newer antiviral combination treatment being able to clear the virus in 45%–80% of cases, often with histologic improvement,94 and on counselling and other interventions (e.g., restriction of alcohol use and other risky practices, hepatitis A and B immunization, treatment of coinfections) reducing disease progression and secondary transmission.63,70,92,93,95,96,97
It is estimated that about one-third of Canadians with chronic HCV infection, or about 90 000 Canadians, remain unaware of their infection.7 For some, HCV infection is diagnosed as a result of a work-up for clinical manifestations or abnormal liver enzyme levels. However, most people are asymptomatic and can be identified only by screening for risk factors (Box 1).
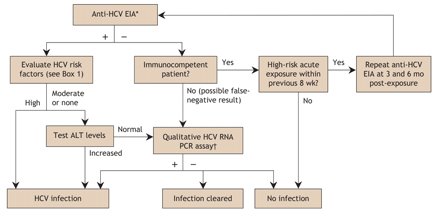
The initial laboratory test is usually an enzyme immunoassay (EIA) for HCV antibodies (anti-HCV) (Fig. 2). The first anti-HCV EIA became available in 1990, but it had relatively poor sensitivity and specificity. In 1992, a second-generation EIA dramatically improved both sensitivity and specificity. The third-generation anti-HCV EIA, in use since the mid-1990s, has a sensitivity of 95%–99% and can detect HCV antibodies 6–8 weeks after exposure.63,98,99 Polymerase chain reaction (PCR) methods detect the presence of HCV RNA much earlier, at 1–3 weeks after exposure.63
Fig. 2: Algorithm for testing for hepatitis C infection.63,93,98 *If the result of the anti-HCV EIA is indeterminate, a qualitative HCV RNA PCR is required. †The threshold for a positive HCV RNA assay result is > 50 IU/mL. HCV = hepatitis C virus, anti-HCV = HCV antibodies, EIA = enzyme immunoassay, ALT = alanine transferase, PCR = polymerase chain reaction.
If the EIA anti-HCV test result is positive, infection can be confirmed with a highly sensitive PCR-based qualitative HCV RNA assay. However, such confirmatory testing is expensive and unnecessary in routine primary care practice. For example, virtually all patients who have a positive anti-HCV test result, a history of risk and abnormal serum alanine transferase values will be viremic (i.e., they will have a positive HCV RNA test result). There is therefore no point in confirming viremia with a HCV RNA test until or unless such a patient is about to start antiviral therapy. Patients who should be tested for HCV RNA include those whose anti-HCV EIA result was inconclusive; immunocompromised patients (e.g., those with HIV or who are undergoing hemodialysis) who may not generate antibodies to infection; patients who are thought to be in a period of acute infection, when the PCR test result will be positive but antibodies have not yet developed; and patients with a positive anti-HCV test result but persistently normal alanine transferase levels. A significant minority of patients who have HCV antibodies and persistently normal liver chemistry test results (especially if alanine transferase values are in the lower half of the normal range) have spontaneously cleared their acute HCV infection but will continue to have detectable HCV antibodies for an indefinite period.63,93,98
Management
If a patient has hepatitis C, look specifically for contraindications to antiviral therapy (Box 2) in the clinical evaluation and for signs of liver disease such as spider nevi and palmar erythema. Jaundice, hepatosplenomegaly, ascites, encephalopathy and gastroesophageal varices are signs of more advanced disease. Baseline laboratory test results should be obtained (Table 2).
Table 2.
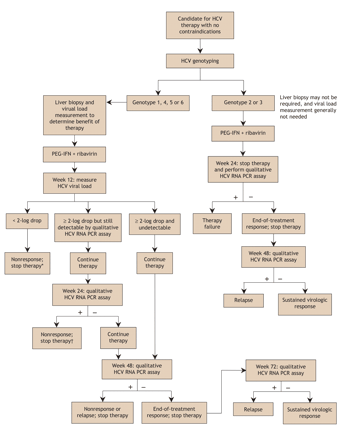
The patient can then be referred to a specialist to assess management options. The complex decision whether to initiate antiviral therapy needs to be based on factors such as the patient's interest, barriers to adherence, clinical and laboratory findings, probability of disease progression without therapy, odds of treatment success, likelihood of adverse effects and absolute and relative contraindications to therapy (Box 2). Genotyping will be performed if the patient is a candidate for antiviral treatment. For patients with genotype 1 HCV, a liver biopsy will usually be arranged if no contraindications exist, and the „viral load” will be measured. Patients with genotype 2 or 3 are expected to have a high likelihood of treatment success (75%–85%) and therefore may not require a liver biopsy and do not require a baseline viral load measurement.63,98 Data are limited for other genotypes because these genotypes are uncommon and because the breakdown of genotypes is not available in the randomized trials conducted in Western countries. Two trials involving patients with genotype 4 in Egypt, where the genotype constitutes up to 90% of HCV infections, have just been published.102,103 This genotype is dominant in the Middle East, and treatment recommendations have recently been published.104 HCV genotype 4 generally responds to treatment as genotype 1 does, and the current recommendation is to treat it in the same manner (48 weeks of therapy).104
Genotype 5 HCV is common in southern Africa and distinctly rare elsewhere. Genotype 6 is generally limited to Southeast Asia. In the absence of clinical trials or even anecdotal series with significant numbers of these genotypes, we recommend treating these genotypes in the same way as genotype 1.
Antiviral treatment
Fig. 3 shows the algorithm for antiviral therapy. In 2004 the Canadian Consensus Conference on the management of viral hepatitis recommended that all patients with hepatitis C be assessed for antiviral therapy.93 This includes patients with sporadically or persistently normal alanine transferase levels. In the past, patients with normal levels had generally not received therapy or even been referred to specialists. However, we now recognize that such patients can have serious underlying liver fibrosis and a treatment response similar to patients with elevated levels. Successful antiviral treatment has been shown to achieve biochemical, viral and histologic responses and to decrease the risk of hepatocellular carcinoma.63,92,95,96,107,108,109,110,111,112 However, as mentioned earlier, there are no prospective data on outcomes following long-term therapy. Sustained virologic response is the primary marker of therapeutic outcome, and it is achieved if serum HCV RNA levels are below the level of detection (≤ 50 IU/mL) 6 months after therapy is completed. As a marker, sustained virologic response strongly predicts viral clearance from the liver, marked histologic improvement and the absence of relapse in the ensuing years.107 In other words, many patients who achieve sustained virologic response appear to be virologically cured.
Fig. 3: Algorithm for hepatitis C antiviral therapy. *If the viral load drops by less than 2 logs compared with that at baseline, therapy can be stopped because the likelihood of achieving a sustained virologic response is less than 3%.63,93,96,105,106 †Therapy should be stopped if the virus is not cleared at 24 weeks, since sustained virologic response is very unlikely.93,107 HCV = hepatitis C virus, PEG-IFN = pegylated interferon, PCR = polymerase chain reaction.
Treatment success rates with antiviral therapy have improved significantly over the past decade. Pegylation of interferon (the addition of inert polyethylene glycol to standard interferon-α) reduces drug clearance rates, which allows dosing to be reduced to one time per week, compared with 3 times per week for standard interferon. Standard interferon-α has a circulating half-life of about 4 hours, and this is dramatically increased to about 80 hours with peginterferon α-2a and about 36 hours with peginterferon α-2b.
Combining weekly subcutaneous peginterferon-α therapy with daily oral ribavirin therapy is more effective than monotherapy with peginterferon or combination therapy with standard interferon and ribavirin. A summary of the results of randomized controlled trials of HCV therapies is presented in Table 3.
Table 3.
Patients must be monitored closely for a variety of adverse effects arising from antiviral therapy (see Box 3). Online Appendix 1, available at www.cmaj.ca/cgi/content/full/cmaj.1030034/DC1, shows the appropriate laboratory investigations during antiviral therapy. Adverse events are more likely with higher medication doses, longer treatment duration and HIV–HCV coinfection. Adverse effects can be sufficiently severe to necessitate discontinuing therapy in up to 17% of patients taking combination peginterferon–ribavirin therapy.63 In particular, ribavirin-induced hemolysis and interferon-induced neutropenia may require dosage adjustments, and hematopoietic growth factors may be used by some experts to treat cytopenias. However, it is unknown whether such growth factors affect treatment outcomes; studies are needed. Patients with depression may benefit from antidepressant and nonpharmacologic therapy. In light of the potential teratogenicity of ribavirin, it is critical that both men and women adhere to strict contraception practices during ribavirin therapy and for 6 months afterward.
Alcohol consumption accelerates the progression of hepatitis C. Avoiding alcohol during therapy is essential because alcohol affects treatment response.63,81,122,123,124,125,126 Strict birth control must be followed by both men and women. Interferon has abortifacient effects in animal studies and should not be used during pregnancy. Ribavirin is embryotoxic and teratogenic. It is not known whether interferon or ribavirin is excreted in human milk. Lactating women should not take interferon–ribavirin combination therapy.
HIV–HCV coinfected patients
HCV and HIV share the same parenteral transmission route. As such, coinfection with both viruses is common.86,127 HCV screening is recommended for all HIV-infected patients. If the patient has a negative anti-HCV test result in the presence of abnormal liver enzyme levels and high hepatitis C risk factors, qualitative HCV RNA PCR testing should be done. Since the introduction of highly active antiretroviral therapy (HAART), HIV-associated morbidity and mortality have dropped drastically, and HCV-associated liver failure and death have become more prominent in coinfected patients.128 HIV accelerates the progression of hepatitis C, especially when the CD4 count is below 200 cells/mL.86,129,130,131 In the presence of HIV, the response to HCV antiviral therapy is not as favourable (sustained virologic response of 14%–38% for genotype 1 and 43%–73% for genotype 2 or 3) and adverse events are more common (Table 3).118,119,120,121 HAART has a favourable effect on the course of hepatitis C, but hepatotoxicity from HAART is a challenge.132,133 In HIV-infected people, HCV seropositivity is an independent predictor of HIV progression and mortality.134,135,136,137,138 Coinfection increases the rates of perinatal transmission of both viruses.50,129
Discussion of the management of HCV infections or HCV– HIV coinfections is complex and beyond the scope of this paper. Consultation with an experienced specialist is recommended, since antiviral therapy is a rapidly changing area. Addiction and mental health comorbidities are important barriers to adherence to treatment and care for some patients. In such circumstances, it is important for primary care providers to enlist the help of colleagues in the fields of social work, addiction or mental health.
Other management issues
Patients with hepatitis C should be warned about the increased risk of disease progression associated with obesity and moderate or heavy alcohol consumption. NSAIDs should be avoided if possible by patients with liver disease, especially those with cirrhosis, because of the risk of acute renal dysfunction. For analgesia, small doses of ASA (< 2 g/d) are vastly preferable to NSAIDs in patients with liver disease. Immunization against hepatitis A and B virus infection if there is no evidence of past infection or immunity93,139,140,141,142 can prevent future assaults on the liver from these infections.
It is unnecessary to screen for HCV-associated hepatocellular carcinoma in the absence of cirrhosis because it is rare that they do not occur together. There have been no randomized controlled trials assessing the value of screening HCV-infected patients for hepatocellular carcinoma. Screening with alpha fetoprotein levels has been shown to range from insensitive (< 20%) to moderately sensitive (> 80%) as the cut-off point decreases from 400 μg/L to 10 μg/L, at the cost of dramatically decreasing specificity.143,144,145,146,147,148,149,150 Screening with ultrasound has varying sensitivities but generally high specificity (> 90%).146,147,151,152 Combining ultrasound with tests for alpha fetoprotein levels may have better sensitivity than either modality alone.147 Results from a cohort study suggest that such combination screening may detect hepatocellular carcinoma at an earlier, resectable stage.153 However, some experts suggest screening only with periodic ultrasounds, since adding a test for alpha fetoprotein levels only marginally improves sensitivity and adds many false-positive test results that need to be intensively investigated.150 Screening for hepatocellular carcinoma after cirrhosis develops may result in earlier detection,58,63,66,154 but whether such screening improves mortality outcomes awaits further prospective randomized controlled trials.
Prevention and counselling
Prevention is an important component of care and should target both HCV-infected people and those at risk but not yet infected. Uninfected people can be counselled to prevent acquiring the virus (see Box 1). Those who are infected can avoid risky practices associated with transmission.
Counselling recommendations include:
• Report newly diagnosed cases of HCV infection to the local public health department in accordance with provincial and territorial requirements.
• Infected patients should not donate blood, organs, tissues or semen, and they should not share sharp items potentially contaminated with blood (e.g., razors, nail clippers, scissors, toothbrushes).
• Infected patients should inform their sexual partners and practise safer sex if they are not in a monogamous, long-term relationship. Those in a monogamous, long-term relationship do not need to change their sexual practices, but they should be informed of the very low risk of sexual transmission except with the moderate-risk sexual activities listed in Box 1. Consider testing the patient's partner for HCV infection.
• Counsel and test HCV-infected patients for HIV and hepatitis B infection.
• Patients should limit their alcohol intake to fewer than 4 drinks per week. The threshold amount of alcohol in HCV-infected patients that increases the fibrosis progression rate remains unclear, but it is almost certainly greater than one daily drink. Therefore, it seems reasonable to set a limit to about half that amount.
• Do not track alanine transferase values. HCV infection causes fluctuations, sometimes marked, in serum levels. They do not have any prognostic significance, and some patients tend to worry unduly about values that are higher than previous ones. We recommend that patients not track these levels.
• Patients should avoid other hepatotoxins, including many herbal products such as kava (a comprehensive list of hepatotoxins is available online as Appendix 2 at www.cmaj.ca/cgi/content/full/cmaj.1030034/DC1).
• No alternative or complementary remedy has been shown to affect HCV liver disease or HCV itself. This includes the „liver herb” silymarin (milk thistle). Therefore, patients should avoid these.
• Hepatitis C can now be treated in many patients. Even without treatment, most patients with chronic infection do not die of liver disease, and most live a normal lifespan.
Conclusions
Although many patients are unaware of their HCV infection status, primary care physicians are in a unique position to help identify, counsel, refer and monitor patients at risk of HCV infection and its associated infections and complications. Ongoing awareness by patients and primary care physicians of factors that promote HCV transmission and disease progression may reduce the burden of disease and identify more patients who would benefit from effective therapy.
Footnotes
-
This article has been peer reviewed.
Contributors: Tom Wong conceived and wrote the article, and Samuel Lee made a substantial contribution to its content. Both authors critically revised the article for important intellectual content and gave final approval of the version to be published.
Competing interests: None declared for Tom Wong. Samuel Lee has been a paid consultant with Roche and Genentech and has received research grants from Roche, Schering–Plough, Migenix, Idenix, Gilead and Novartis as well as speaker fees and educational grants from Roche and Axcan.
REFERENCES
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.↵
- 90.↵
- 91.↵
- 92.↵
- 93.↵
- 94.↵
- 95.↵
- 96.↵
- 97.↵
- 98.↵
- 99.↵
- 100.
- 101.
- 102.↵
- 103.↵
- 104.↵
- 105.↵
- 106.↵
- 107.↵
- 108.↵
- 109.↵
- 110.↵
- 111.↵
- 112.↵
- 113.
- 114.
- 115.
- 116.
- 117.
- 118.↵
- 119.↵
- 120.↵
- 121.↵
- 122.↵
- 123.↵
- 124.↵
- 125.↵
- 126.↵
- 127.↵
- 128.↵
- 129.↵
- 130.↵
- 131.↵
- 132.↵
- 133.↵
- 134.↵
- 135.↵
- 136.↵
- 137.↵
- 138.↵
- 139.↵
- 140.↵
- 141.↵
- 142.↵
- 143.↵
- 144.↵
- 145.↵
- 146.↵
- 147.↵
- 148.↵
- 149.↵
- 150.↵
- 151.↵
- 152.↵
- 153.↵
- 154.↵