Enhancement of patient safety has become a priority for health care practitioners and organizations. Adverse medication events remain a major concern, as drug error is a significant cause of adverse outcomes for hospital inpatients.1 Patients are particularly vulnerable to medication error during the perioperative period. The leading cause of malpractice suits for Canadian anesthesiologists is medication error, and misidentification of drugs is the most frequent underlying problem.2,3
Improved safety requires a team effort with a focus on patients' well- being.4 Thus, it is disturbing that AstraZeneca has marketed in Canada a product that fails to meet the minimum labelling standard set out in the Food and Drugs Act.
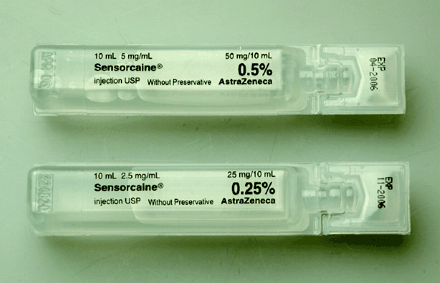
Bupivacaine is a potent, potentially lethal local anesthetic that is used for local infiltration and for spinal and epidural anesthesia. It is considerably more cardiotoxic than many other local anesthetics. The label on the bupivacaine Polyamp® ampoule sold by AstraZeneca does not include the generic name of the drug, but rather identifies the product only by the brand name, Sensorcaine (Fig. 1).5
Polyamp® ampoules containing bupivacaine are labelled with the company's brand name, Sensorcaine, rather than the generic name, bupivacaine.
The Food and Drugs Act states that “the inner and outer labels of a drug shall show (i) the proper name, if any, of the drug which, if there is a brand name for the drug, shall immediately precede or follow the brand name in type not less than one-half the size of that of the brand name; (ii) if there is no proper name, the common name of the drug.” The act also specifies that “No person shall sell a drug that is not labelled as required by these Regulations.”6
Clearly, the Sensorcaine packaging does not meet these legal requirements. This situation raises several disturbing questions. Why would an international pharmaceutical firm design ampoule labels with an emphasis on marketing rather than patient safety? How could this product bypass scrutiny by Health Canada and be introduced into Canadian hospitals? Once the oversight was brought to the attention of AstraZeneca, why did the manufacturer not post warnings and apply additional adhesive labels to the ampoule until a new product, appropriately labelled, was available?
Physicians and health care providers must demand that the pharmaceutical industry “join the team” and make patient safety more important than marketing considerations.