Abstract
The pathogenic, diagnostic and therapeutic landscape of sepsis is no longer confined to the intensive care unit: many patients from other portals of entry to care, both outside and within the hospital, progress to severe disease. Approaches that have led to improved outcomes with other diseases (e.g., acute myocardial infarction, stroke and trauma) can now be similarly applied to sepsis. Improved understanding of the pathogenesis of severe sepsis and septic shock has led to the development of new therapies that place importance on early identification and aggressive management. This review emphasizes approaches to the early recognition, diagnosis and therapeutic management of sepsis, giving the clinician the most contemporary and practical approaches with which to treat these patients.
Severe sepsis and septic shock are common, accounting for about 2.9% of hospital admissions and 10% of admissions into the intensive care unit (ICU). The mortality rates for these 2 conditions exceed 30%.1 A report from an attempt to quantify the incidence of sepsis upon initial arrival at hospital2 estimated that half of such cases come to the emergency department (ED), which would mean some 387 600 cases in US EDs annually. A retrospective observational study3 of 496 patients with severe sepsis and septic shock who were admitted to several Canadian ICUs found that 32% had been admitted through their respective EDs.
The importance of the first 6 hours
Patients admitted and treated for sepsis in the ICU are often transferred from general medical–surgical practice units (GPUs), operating rooms (ORs), EDs, long-term care facilities and other hospitals. The diagnosis and treatment of these patients may be suboptimal, even among those who were admitted to GPUs4 or the ICU.5 Delays in the identification, transfer and management of critically ill patients during the first 6 hours after ICU admission have been associated with higher mortality rates4 and increased utilization of hospital resources.6 Within the last 5 years, advances in the treatment of severe sepsis and septic shock have provided new therapies to treat this disease. Although these studies were ICU-based, the timeliness of treatment became a more important issue when Rivers and colleagues7 were able to show a significant mortality benefit when hemodynamic optimization was provided within the first few hours of disease presentation. This “golden hour” and “silver day”8 perspective of early resuscitation, which traditionally has been applied to trauma, can now be applied to severe sepsis and septic shock. Early diagnosis and rapid intervention became synonymous with improved outcomes for trauma patients, which inspired the concept of the “golden hour.”9 In turn, the “silver day” represented the first day's remaining hours, during which aggressive correction of shock and organ dysfunction was found to decrease health-care resource utilization10 and improve outcomes.8 These ideals have been incorporated into the Surviving Sepsis Campaign, a multinational initiative, which recommends a 24-hour sepsis pathway that includes a critical 6-hour course of action.11
The transition from sepsis to severe sepsis
The initial presentation of severe sepsis and septic shock is often nonspecific, and its severity, cryptic. Patients who arrive with a relatively benign or clinically unapparent infection can progress within hours to a more devastating form of disease.
Abnormalities in temperature, heart and respiratory rates and leukocyte count are manifestations of the systemic inflammatory response syndrome (SIRS).12,13 Because SIRS is a host response and can also arise from noninfectious causes, as a disease stage it is nonspecific. Sepsis is defined by the presence of 2 or more SIRS criteria in the setting of a documented or presumed infection. Severe sepsis is hallmarked by concomitant organ hypoperfusion or organ dysfunction (Box 1).7,14 Septic shock results when blood pressures fall and patients become hypotensive (i.e., < 90 mm Hg systolic blood pressure or < 65 mm Hg mean arterial pressure) despite adequate fluid resuscitation, and the patient requires vasopressor support.
The transition from sepsis to septic shock occurs most often during the first 24 hours of hospitalization. It carries with it an increase not only in morbidity10 but also in mortality: 20%–46%.7,15,16,17 The decreases in tissue oxygen delivery and the cardiovascular insufficiency that accompany this transition may not be detected by vital signs nor SIRS criteria.18 It is at this critical juncture that outcomes can be much improved.
The science
Host response to infection
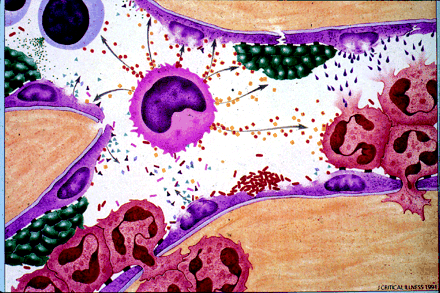
Numerous and complex pathogenic mechanisms are responsible for the transition from sepsis to severe sepsis and septic shock. The initial host response to infection is a humoral, cellular and neuroendocrine reaction to the infectious insult. Cells such as neutrophils, monocytes, macrophages, basophils and platelets interact with endothelial cells by means of adhesion molecules, receptors, selectins, immunoglobulins, oligosaccharides and integrin families.19 Endothelial interaction involving these mediators and products of inflammation is further amplified when the coagulation and complement systems are activated. In the most exaggerated scenario, vasoregulatory dysfunction and microaggregation impair microvascular flow, creating local ischemia and hypoxia, which may impair cellular respiration.20,21,22,23,24,25,26,27 Structural decreases in the permeability of the endothelium permit inflammatory cells and products to leave the circulation (Fig. 1),28 leading to generalized edema.
Fig. 1: Endothelial disruption and the inflammatory response. The platelets (green bodies), endothelial cells (purple), macrophages (light blue cell with dark centres), polymorphonuclear cells (pink with 3 inner lobes) and cytokines (small pellets) are inflammatory mediators. Adapted with permission from Matuschak GM. Continuous central venous and pulmonary artery oxygen saturation monitoring in the critically ill. Crit Care Med 1996;24:1769-71.28
Since endothelium is ubiquitous throughout the body, this “pan-endothelial cell disruption” is central to the development of multisystem organ failure. In recent years, components of this host response have been the target of outcome trials for many therapies.
Oxygen transport and utilization
Impairment in oxygen delivery and utilization at the tissue level parallels the host response to infection; this pathogenic mechanism leads to global tissue hypoxia. Tissue hypoxia not only results from host inflammatory response, it can also stimulate further inflammation.22 Fundamental to the recognition and treatment of global tissue hypoxia is an understanding of the principles of oxygen transport and utilization.
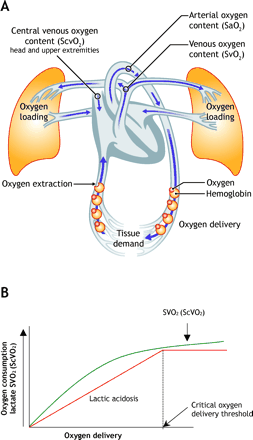
Oxygen is delivered to the tissues as a product of cardiac output (heart rate ∞ stroke volume) and oxygen content: (hemoglobin oxygen saturation ∞ hemoglobin ∞ 1.34) + (partial pressure of oxygen ∞ 0.003). The tissues extract a percentage of the delivered oxygen for cellular respiration: oxygen consumption. The blood, with its remaining oxygenation, returns to the venous circulation (Fig. 2); its remaining oxygenation can be measured with a blood sample from the pulmonary artery (mixed venous oxygen saturation, SvO2) or from the central venous circulation (central venous oxygen saturation, ScvO2).
Fig. 2: Oxygen transport and utilization.
SvO2 is not readily measurable without a Swan–Ganz catheter. However, ScvO2 is measurable through central venous cannulation of the superior vena cava or right atrium (Fig. 2). SvO2 measurement indicates the level of venous oxygenation throughout the body; in nonshock states, it generally reflects venous oxygenation from the head and upper extremities. When the patient is in shock and SvO2 testing yields low values, ScvO2 measurements will consistently read (5-6%) higher but still correlate well with SvO2.29,30,31
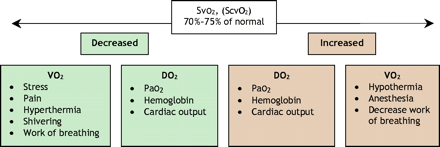
Global tissue hypoxia develops when systemic oxygen delivery is insufficient to meet the oxygen demands of the tissues. When this critical delivery threshold is not reached, increased lactate production ensues as a by-product of anaerobic cellular respiration. A low SvO2 (< 65%) or ScvO2 (< 70%) result and an increased serum lactate concentration suggest the presence of global tissue hypoxia because a greater percentage of delivered oxygen is being extracted by the tissues, resulting in less venous oxygen to be measured distal to this extraction. This is considered to be early-stage or hypodynamic sepsis.32 A normal SvO2 or ScvO2 and serum lactate level suggest that oxygen supply meets demand (Fig. 3 and Fig. 4). Correction of these hemodynamic perturbations with prompt resuscitation, or in the presence of adequate compensatory mechanisms in the host, may result in a hyperdynamic state with high cardiac output. Although the origin of lactate generation in sepsis has been debated,33 its presence and clearance over time are strongly associated with morbidity and morality.34,35
Fig. 3: The clinical utility of venous oxygen saturation (SvO2 or ScvO2).
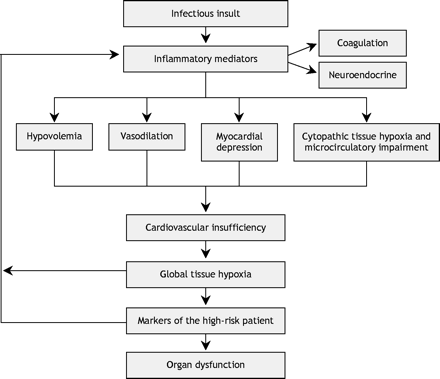
A normal or increased SvO2 or ScvO2 value in isolation does not always equate with normal or adequate tissue oxygenation: these measurements reflect global oxygen kinetics rather than those at the tissue level. In cases of severe sepsis or septic shock, abnormalities of hypoperfusion can still exist even in the presence of normal or high cardiac output and blood pressure. This “distributive shock” refers to a state of either regional or systemic hypoperfusion secondary to derangements in blood flow or the loss of vasoregulatory control in microcirculatory vascular beds.16,36,37 In particular, a normal or high SvO2 or ScvO2 reading accompanied by an increased blood lactate concentration (in other words, increasing metabolic acidosis) indicates that despite adequate global systemic oxygen delivery, the tissues are unable to extract the oxygen, perhaps because of microvascular shunting, microcirculatory failure38 or mitochondrial dysfunction. This clinical syndrome has been termed cytopathic tissue hypoxia,39 an oxygen delivery– independent state that is associated with increased morbidity and mortality (Fig. 4).40,41
Fig. 4: The pathogenesis of cardiovascular insufficiency.41
Global tissue hypoxia has pathogenic implications. In vitro, there is a significant correlation between SvO2 and impairment of mitochondrial oxygen utilization.42 The resultant tissue hypoxia can serve to further activate endothelial mediators,22 causing loss of vascular integrity,43 increased release of inflammatory cytokines44 and procoagulants,45 and reduced fibrinolysis.46
The microcirculation
Much of the pathophysiology of sepsis involves disordered microcirculation. Considerable research has been conducted to enable clinicians to assess metabolic by-products and be able at bedside to envision the patient's microcirculation.38,47 Intestinal and sublingual capnography devices, which measure partial carbon dioxide pressures (PCO2) in the stomach and under the tongue, respectively, may reflect local cellular respiration in those tissues, suggesting regional hypoperfusion.48,49,50
A difference in mixed venous– arterial PCO2 measurements between sampling locations correlates inversely with the patient's cardiac index. In a prospective cohort study, the venous–arterial PCO2 differences obtained from the pulmonary artery and central venous circulations were equal and inversely correlated with the cardiac index. A venous– arterial PCO2 difference of more than 5 mm Hg suggests that cardiac output to tissue is inadequate and a supply-dependent state of severe sepsis exists. Hence, in the absence of a Swan– Ganz catheter, this value may be used as an additional surrogate marker that may reflect adequacy or inadequacy of cardiac output.51
Direct methods to evaluate microcirculatory flow are intravital microscopy (IVM) or orthogonal polarization spectral (OPS) imaging.38 These tools are not yet available for widespread clinical use, but are currently undergoing evaluation; future research should help determine their role in sepsis treatment.
The clinical link
Identification of the high-risk patient
Early identification of the high-risk patient begins with a marker of illness severity. The systemic inflammatory response syndrome (SIRS) can be invoked by infection, trauma, ischemic or reperfusion injury, or sterile inflammation.52 Although the components of SIRS are nonspecific (Table 1), the combination of a suspected infection and the presence of SIRS may help alert the clinician to a possible diagnosis of sepsis. Although hypotension is another clinical sign that may signal the onset of septic shock, patients may present with severe sepsis and clinically significant global tissue hypoxia in its absence. Hence, signs of organ hypoperfusion and organ dysfunction (Table 1) must be sought by the treating clinician to evaluate severity of illness.
Table 1.
Because significant global tissue hypoxia in patients with sepsis can coexist with vital signs within normal ranges,53 metabolic markers may help to identify high-risk patients. A single lactate measurement of 4 mmol/L or more at initial presentation is associated with an increased rate of mortality.54,55 Failure to clear lactate levels during as little as the first 6 hours after presentation is also associated with higher morbidity and mortality.35 Serum bicarbonate measurements and arterial base deficits correlate and remain indicators of tissue hypoperfusion.56 However, serum bicarbonate may be depressed and the base deficit persist when large volumes of chloride-rich crystalloid resuscitation fluids are administered.57
Serologic tests and biomarker assays may one day be critical in the assessment and treatment of the patient with sepsis.58 Likely candidates include C-reactive protein (CRP),59,60,61,62,63 endotoxin (a component of Gram-negative cell walls),58,64 brain natriuretic peptide (an indicator of myocardial dysfunction),65,66,67 procalcitonin,68,69,70,71,72,73,74,75 interleukin-6 and endogenous protein C.76,77,78,79,80,81 At present their use is limited because these assay results as yet lack diagnostic accuracy, prognostic capability and timeliness; but one day, these markers may provide bedside tests to help diagnose and treat the entire spectrum of sepsis disease.
Several scoring systems have been developed to determine severity of illness in the ICU and predict the risk of death in populations of critically ill patients. These include the Acute Physiology and Chronic Health Score (APACHE) II and III,82 Simplified Acute Physiology Score (SAPS),83 Sepsis-related Organ Failure Assessment Score,84 Multiple Organ Dysfunction Score,85 Logistic Organ Dysfunction System86 and Mortality Probability Models.87 APACHE II and SAPS II scores can readily be calculated with use of the Internet; at www.sfar.org/scores2/apache22.html and www.sfar.org/scores2/saps2.html a clinician can use these tools to compare illness severity and, moreover, access guidelines for diagnostic prognostication and therapeutic interventions, such as the use of recombinant human activated protein C (r-APC). The MEDS score, developed specifically for sepsis patients in the ED, correlates with sepsis outcomes.88 In this scoring system, independent predictors of mortality upon arrival at the ED include terminal illness, tachypnea, hypoxia, septic shock, thrombocytopenia, a band proportion above 5%, infection of the lower respiratory tract, residence in a nursing home and altered mental status.88 Current Canadian guidelines are evolving for triage and the use of acuity scales; future adjustments will be required to better detect sepsis in its early stages.89
From a therapeutic perspective,90 Hillman91 and others92,93,94,95,96,97,98,99,100,101,102 examined the effects of an in-hospital medical emergency team using early identifiers of the high-risk patient. These teams of intensive-care nurses and physicians were mobilized when specified alterations in airway, breathing, circulation and neurologic status were met. Whether patient outcomes improve with this approach remains inconclusive.
Appendix 1 lists several useful Web resources related to sepsis care.
Treatment
Early antimicrobial therapy
The association of timely and appropriate antibiotic therapy with improved morbidity and mortality has been established in the ICU setting.103,104 Observational studies105,106 suggest a significant reduction in mortality when antibiotics are administered within 4105 and 8 hours106 of hospital presentation (p < 0.01). Current Surviving Sepsis Campaign recommendations11 are to administer antibiotics within 1 hour of a sepsis diagnosis.
Specific antibiotic strategies are beyond the scope of this paper, and reviews on antibiotic strategies may be found elsewhere.107 However, we recommend broad coverage initially that is tailored to the potential source of infection and according to local hospital sensitivity and resistance patterns.
Surgical consultation for source control is appropriate when a patient has an undrainable abscess or an intra-abdominal source of sepsis. Consideration should also be given to the possibility of resistant organisms when patients live in nursing home or are users of intravenous drugs.
Early hemodynamic optimization
Resuscitation strategies for cases of severe sepsis or septic shock have been studied intensely and debated for years.108 Investigations109 involving strategies aimed at obtaining supranormal physiologic endpoints in ICU patients up to 72 hours into their hospitalization have led to negative and even deleterious results. Meta-analyses of sepsis resuscitation trials have indicated that early interventions110 that take place before organ dysfunction111 provide better outcomes. A recent trial7 involving ED patients with severe sepsis or septic shock to examine hemodynamic resuscitation to normal physiologic parameters or early goal-directed therapy (EGDT) revealed a statistically significant mortality reduction of 16.5%.
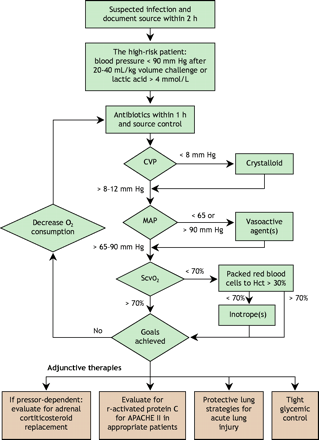
EGDT is an algorithmic approach of hemodynamic optimization (Fig. 5) that aims to restore the balance between oxygen supply and demand in cases of severe sepsis or septic shock within the first 6 hours of ED care. The strategy targets the attainment of adequate oxygen delivery by optimization of intravascular volume (preload) with the use of central venous pressure (CVP) monitoring, blood pressure (afterload) with mean arterial pressure monitoring, contractility with use of monitoring to avoid tachycardia, and restoration of the balance between systemic oxygen delivery and oxygen demand (guided by ScvO2 measurements) to resolve global tissue hypoxia. The components of EGDT were derived from the recommendations by the Society of Critical Care Medicine for hemodynamic support in sepsis.112,113
Fig. 5: Treatment options in sepsis. CVP = central venous pressure, MAP = mean arterial pressure, ScvO2 = central venous oxygen saturation, Hct = hematocrit.
Hemodynamic monitoring
Early hemodynamic optimization requires the monitoring of CVP, arterial blood pressure and ScvO2. Intra-arterial pressure monitoring is recommended — especially for patients who are prescribed vasopressor medications,11,113 but with the caveat that vasopressor agents may cause central arterial pressure to be underestimated when measured from the radial artery.114 ScvO2 can be measured intermittently from venous gas samples taken from the distal port of a standard central venous catheter or continuously by use of a fibre-optic central venous catheter and monitor (Edwards Lifesciences, Irvine, Calif.). Although in expert hands the pulmonary artery remains effective as a measurement site, proof of outcome benefit from its use remains to be demonstrated.115,116,117
Volume therapy
The first goal of EGDT in cases of sepsis is repletion of the patient's intravascular volume. Intravenous fluid therapy should begin with rapid and repeated 500-mL boluses of either crystalloid or colloid fluid up to an initial resuscitation volume of 20– 40 mL/kg body weight, to attain a CVP of 8–12 mm Hg.
Until last year no randomized controlled trial or systematic review has definitively shown a benefit in the critically ill for the use of colloid or crystalloid fluid.118,119,120 However, a large randomized controlled trial119 that compared 4% albumin with normal saline in the treatment of 6997 heterogeneous critically ill patients in need of volume resuscitation found no significant difference in mortality between the groups. Although a subgroup analysis of patients with severe sepsis suggested a trend for mortality benefit for the group receiving albumin, these findings should be considered only for the generation of hypotheses: they require confirmation with a randomized controlled trial involving patients with sepsis.
Vasoactive agents
After the CVP goal is met, a vasopressor drug is given if the patient remains hypotensive (mean arterial blood pressure < 65 mm Hg). The target goal of a mean arterial pressure of 65 mm Hg has been shown to be physiologically equivalent to higher pressures.121,122
Vasopressor agents include dopamine (5–20 μg/kg/min intravenously), norepinephrine (2– 20 μg/min), phenylephrine (40– 300 μg/min) and vasopressin (0.01– 0.04 units/min). Both norepinephrine and dopamine have been advocated as first-line vasopressor agents in cases of sepsis.113 Because tachycardia may be exacerbated by β-agonist vasopressors, agents with more α-agonist effects (i.e., norepinephrine and phenylephrine) may be preferable for patients with pre-existing tachycardia or coronary disease.
When hypotension persists in a patient already taking a vasopressor medication, vasopressin deficiency may be considered;123,124 vasopressin is an endogenously produced hormone that is deficient in many patients with septic shock. Exogenously administered vasopressin in physiologic replacement doses (0.01–0.04 units/min) may act synergistically with other vasopressor agents, and has been associated with early withdrawal of other catecholamines.125,126,127 Current treatment doses of 0.01–0.04 units/min are meant to reflect physiologic replacement doses. High doses of 0.06–1.8 units/min (as traditionally used)128,129 are not recommended in the context of septic shock because of reported adverse events.130,131
Epinephrine (1–10 μg/min) may be considered for patients unresponsive to other vasopressors. It increases mean arterial pressure by increasing cardiac output and stroke volume.132
Deleterious effects associated with vasopressor agents include the development of splanchnic hypoperfusion, excess tachycardia and coronary ischemia.133 Existing evidence has not definitively proven one vasopressor agent superior to another in the setting of severe sepsis or septic shock.133 Some definitive evidence on the current role of vasopressin and its effect on outcome is expected from the Canadian multicentre Vasopressin and Septic Shock Trial, VASST.
Administration of erythrocytes
If the ScvO2 remains below 70% after optimization of preload, afterload and arterial oxygen saturation, the patient's oxygen-carrying capacity may be augmented by the administration of packed erythrocytes to achieve a hematocrit above 30%. Although recent studies134,135 have suggested that more conservative transfusion thresholds may be tolerated in a heterogeneous and clinically stable group of critically ill patients, these results should not be extrapolated to acutely septic patients with an oxygen supply–demand mismatch. In the acute phase of resuscitation a hematocrit target of 30% appears reasonable, with a more restrictive transfusion strategy in the convalescent phase of the disease. Resuscitation trials that specifically address questions such as optimal erythrocyte transfusion triggers and the value of fresh versus old blood should help to further define the optimal role of red blood cells for patients with early septic shock.
Inotropic therapy
Sepsis may be accompanied by myocardial suppression in 10%– 15% of patients, irrespective of age.7,136 In the EGDT study,7 these patients had persistently low readings of ScvO2 after meeting the goals of CVP, mean arterial pressure and hematocrit. Some of them presented with an initially elevated CVP as a result of decreased ventricular compliance rather than volume overload. Inotropic support with dobutamine may improve myocardial depression, but may also unmask underlying hypovolemia because it acts to increase contractility and reduce peripheral vascular resistance. As ventricular compliance and contractility respond, CVP will decrease as the stroke volume improves.137,138 Further fluid replacement may be required to maintain CVP at 8– 12 mm Hg. Dobutamine is then titrated at increments of 2.5 μg/kg/min every 20–30 minutes to a ScvO2 measurement of 70%.
Clinicians must take care to avoid tachycardia (by keeping the heart rate < 100 beats/min) to optimize stroke volume and minimize myocardial oxygen consumption. Milrinone, a phosphodiesterase inhibitor, may be considered as an alternative agent to augment cardiac output. Like dobutamine, it is an inotropic agent that also reduces peripheral vascular resistance. However, its half-life (2.4 hours) is much longer than that of dobutamine, and it accumulates in renal failure. Future resuscitation research aimed specifically at optimal inotropic support is required to further define the role of these agents.
Decreasing oxygen consumption
In the severely septic patient, maximized oxygen delivery may be inadequate to restore the balance between oxygen supply and demand. Strategies to minimize oxygen demand should therefore also be considered. Intubation, sedation and analgesia with mechanical ventilation will reduce both the work of breathing and oxygen consumption by the respiratory muscles.139 Control of fever with antipyretics such as acetaminophen will also decrease oxygen consumption.
Adjunctive therapies
Several additional therapies initiated within the first 24 hours after identification of severe sepsis and septic shock have recently demonstrated a mortality benefit. Early implementation of these therapies may improve patients' survival, since they may wait several hours or even days before transfer to an ICU.
Steroid therapy
In the neurohumoral response to septic shock, many patients have manifestly inadequate adrenal reserve, or relative adrenal insufficiency (RAI).140,141,142,143 The mechanism for RAI is complex and incompletely understood, but is likely caused in part by the inflammatory cascade leading to an inadequate release or response to adrenocorticotropin, in combination with peripheral steroid resistance at the receptor level.144,145 RAI should be considered clinically distinct from absolute adrenal insufficiency because RAI usually clears up when the septic shock resolves. Patients with RAI therefore do not require corticosteroid replacement after resolution of shock.
Compared with placebo, the administration of low doses of hydrocortisone to patients with septic shock decreased their requirements for vasopressors146,147 and lowered their mortality rate.148 In the one multicentre, randomized controlled trial that demonstrated a mortality benefit,117 corticosteroids (hydrocortisone 50 mg intravenously every 6 hours and fludrocortisone 50 μg by mouth once daily) were started within 8 hours after the diagnosis of septic shock and continued for 7 days. There was a 10% absolute reduction in 28-day mortality in the group that received hydrocortisone and fludro-cortisone and who were diagnosed with RAI at randomization. In this study,117 RAI was diagnosed if the patient's increase in serum cortisol 1 hour after he or she received 250 μg of adrenocorticotropin was 250 nmol/L (9 μg/dL) or less. However, the study was unable to determine if the administration of corticosteroids was safe and effective for the group of patients who did not have RAI according to the definition used in the study by Annane and associates.148 Since the cut-off values for benefit are still in question, it should be left to the treating clinician to decide whether to order an adrenocorticotropin stimulation test to aid clinical decision-making. Because dexamethasone does not interfere with adrenocorticotropin test results, immediate empiric treatment with 2 mg of this steroid may be given and the test performed at a more convenient time.
Although the study by Annane and associates148 has helped to establish the use of low-dose corticosteroids to treat septic shock, several questions remain. These include better consensus definitions for RAI with regard to baseline and stimulated cortisol concentrations in septic shock, and whether the measurement of free cortisol may increase the accuracy of adrenocortical function tests.149,150 Much debate has ensued on the correct definition of RAI; it is still unclear whether patients who were deemed to not have RAI in the Annane study would have benefited from hydrocortisone.150 Future research in this area will help to answer these questions more definitively.
Activated protein C
Protein C is an endogenous anticoagulant that also possesses profibrinolytic, anti-inflammatory anti-apoptotic effects and may improve microcirculatory flow.76,151 The Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study,76 a multicentre randomized controlled trial, showed that the administration of r-APC (also known as drotrecogin alfa [activated] or Xigris) reduced mortality from severe sepsis or septic shock by 6% compared with placebo.76,152,153,154 Subgroup analyses suggested that survival with r-APC was improved in patients whose APACHE II scores were 25 or greater and also had dysfunction of 2 or more organs. Receiving r-APC was also associated with a trend toward an increased risk of bleeding (3.5% v. 2.0%, p = 0.06). In the PROWESS trial, r-APC was started within 24 hours after the criteria for severe sepsis were met.
Our understanding of the role of r-APC in earlier phases of disease presentation, such as those seen in the ED, is evolving.155 Post-hoc studies conducted on subsets of patients participating in r-APC trials suggest that earlier administration of r-APC may improve morbidity and mortality and moreover reduce health care resource consumption.155 A second large randomized controlled trial156 that compared the efficacy of r-APC with that of placebo in patients with less severe sepsis (defined as zero or one organ dysfunction) was terminated early, when there appeared to be no mortality benefit for this patient population. This study did not address the timing of administration. Future research efforts, exploring the optimal timing for administration of r-APC and other anticoagulants will help determine its role in the earlier stages of severe sepsis and septic shock.
Protective lung strategies
Experimental data have revealed that mechanical ventilation with large tidal volumes can stretch lung tissue to excess (volume trauma), exacerbating the inflammatory response and leading to acute lung injury.157 A multicentre randomized controlled trial125 involving patients with acute lung injury and adult respiratory distress syndrome (ARDS) evaluated the effect of a low tidal volume (6 mL/kg of estimated body weight) versus a conservative volume strategy (12 mL/kg) with plateau pressures maintained at < 30 cm H2O and demonstrated a 9.9% absolute reduction in 28-day mortality in the low tidal volume group for patients with acute lung injury or ARDS. Since sepsis is the most common cause of ARDS, it is prudent to institute ventilation with low tidal volumes when lung injury or ARDS is present. Prophylactic strategies to prevent the development of ARDS have not yet been researched, although a Canadian trial, the Lung Open Ventilation Study (LOVS), is currently evaluating the efficacy of recruitment maneuvers to keep the lungs open in cases of ARDS. LOVS will provide further information on optimal mechanical-ventilation methods for patients who have septic shock and ARDS.
Tight glycemic control
Another therapeutic strategy that has been found to confer a mortality benefit to critically ill and predominantly postsurgical cardiothoracic patients is tight glycemic control.126 When conservative (10.0–11.1 mmol/L) glycemic control was compared with tight control (4.4– 6.1 mmol/L) in a multicentre, randomized controlled trial,126 tight control led to a significant reduction in mortality (8.0% v. 4.6%, p < 0.04) and improved morbidity at 12 months.158 This included a significant reduction in organ failure (p = 0.04) with a proven septic focus. Studies are underway in Australia, New Zealand, Britain and Canada that should help to determine whether tight glycemic control affords a mortality benefit for the more severely ill among critically ill patients.
Future directions
Resuscitation research is required to further elucidate the role of established resuscitation treatments for septic shock, such as fluid therapy, vasopressors, inodilating agents and erythrocyte transfusion triggers. New research on the diagnostic and prognostic roles of monitoring microcirculation, sepsis biomarkers and scoring tools for severity of illness is underway that may help in the rapid identification, diagnosis and management of these patients.
Other innovative therapies may eventually play a role in the treatment of sepsis. Examples include the administration of vasodilators, high-volume hemofiltration and corticosteroids for severe community-acquired pneumonia. In an early stage of severe sepsis, a patient may have normal or increased blood pressure after volume resuscitation despite signs of tissue hypoperfusion.18 Low-dose nitroglycerin (5–50 μg/min) may improve ScvO2 by decreasing afterload when the mean arterial pressure is elevated, especially after volume resuscitation. Preliminary data also suggest that nitroglycerin may improve microcirculatory blood flow by allowing for capillary recruitment and increased perfusion.159 High-volume hemofiltration with removal of inflammatory cytokines is another therapeutic strategy that may afford a mortality benefit for patients with early severe sepsis or septic shock.160
In a recent preliminary multicentre trial, patients admitted to ICU with severe community-acquired pneumonia received protocol-guided antibiotic treatment and were randomly assigned to hydrocortisone infusion or placebo groups. Hydrocortisone was given as an intravenous 200-mg bolus followed by infusion at a rate of 10 mg/h for 7 days. The hydrocortisone group had significant improved chest radiograph scores (p < 0.001), C-reactive protein levels (p = 0.01), Multiple Organ Dysfunction Scores (p = 0.003) and delayed septic shock (p = 0.001). Hydrocortisone treatment was associated with shorter hospital stays (p = 0.03) and reduced mortality (p = 0.009).161 Future research shall help to determine if there may be roles for these therapies in the clinical arena.
Conclusion
Early recognition and prompt resuscitation during the first several hours of severe sepsis and septic shock optimizes outcome. Other therapies that can afford a mortality benefit for patients with severe sepsis and septic shock include the administration of corticosteroids, activated protein C, mechanical ventilation with low tidal volumes, and tight glycemic control. Specific emphasis on appropriate triage to ensure prompt diagnosis of the high-risk patient is vital to the launch of a coordinated and cooperative effort by the primary treating clinician and the intensivist. Because the reversibility of this disease and the resultant mortality may be greatest during the earliest stages of presentation, proper sepsis management should not be confined within the walls of an ICU.
Footnotes
-
This article has been peer reviewed.
Contributors: All the authors contributed to the drafting and critical revision of the manuscript, gathered the information presented, and approved the final version for publication.
Competing interests: None declared by David Morro and Kandis Rivers. Emanuel Rivers has received grants from the Lilly Corporation, Edwards Lifesciences and Elan Pharmaceuticals. Lauralyn McIntyre has received grants from Edwards Lifesciences, Abbott Laboratories, Bristol-Myers Squibb and Spectral Diagnostics.
REFERENCES
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.↵
- 90.↵
- 91.↵
- 92.↵
- 93.↵
- 94.↵
- 95.↵
- 96.↵
- 97.↵
- 98.↵
- 99.↵
- 100.↵
- 101.↵
- 102.↵
- 103.↵
- 104.↵
- 105.↵
- 106.↵
- 107.↵
- 108.↵
- 109.↵
- 110.↵
- 111.↵
- 112.↵
- 113.↵
- 114.↵
- 115.↵
- 116.↵
- 117.↵
- 118.↵
- 119.↵
- 120.↵
- 121.↵
- 122.↵
- 123.↵
- 124.↵
- 125.↵
- 126.↵
- 127.↵
- 128.↵
- 129.↵
- 130.↵
- 131.↵
- 132.↵
- 133.↵
- 134.↵
- 135.↵
- 136.↵
- 137.↵
- 138.↵
- 139.↵
- 140.↵
- 141.↵
- 142.↵
- 143.↵
- 144.↵
- 145.↵
- 146.↵
- 147.↵
- 148.↵
- 149.↵
- 150.↵
- 151.↵
- 152.↵
- 153.↵
- 154.↵
- 155.↵
- 156.↵
- 157.↵
- 158.↵
- 159.↵
- 160.↵
- 161.↵