- © 2004 Canadian Medical Association or its licensors
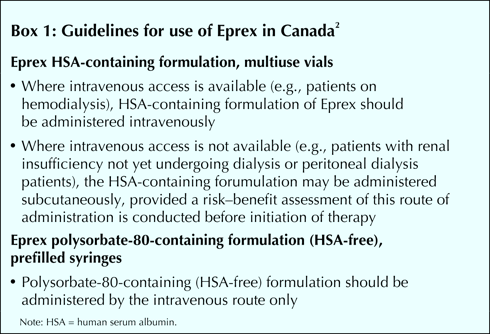
We are writing to correct and clarify several points in Barbara Sibbald's article on recombinant human erythropoietin (epoetin alfa [Eprex]).1 Sibbald erroneously states that Health Canada has advised practitioners “against intravenous injection” of the drug “for patients with chronic renal failure.” In fact, Health Canada's advisory of Jan. 13, 2004, recommended that “where intravenous access is available, Eprex HSA-[human serum albumin] containing formulation should be administered intravenously”; where intravenous access is not available, the HSA-containing formulation may be administered subcutaneously but only after a risk–benefit assessment.2 These guidelines for the use of Eprex in Canada are detailed in the text box.
Sibbald correctly communicated the well-documented risk of pure red cell aplasia (PRCA) associated with the use of Eprex but failed to note that the degree of risk differs with the formulation and route of administration. Two formulations are available in Canada, one containing HSA as the stabilizer, the other containing polysorbate-80 (i.e., HSA-free [not “HSA-3,” as mentioned in the CMAJ article]). The latter has recently been presented in a prefilled syringe intended for subcutaneous administration, whereas the formulation containing HSA is presented in multiuse vials. Most cases of PRCA are associated with HSA-free Eprex administered subcutaneously (this route is associated with increased development of antibodies to an immunogen3). We are not aware of any domestic or foreign reports of PRCA associated with Eprex administered intravenously.
In Europe, Eprex is available only in the HSA-free formulation. Furthermore, contraindications are not absolute in Europe, so use of a “contraindicated” product is not actually prohibited. Therefore, it was appropriate for the European Medicines Agency to issue an advisory to health care practitioners contraindicating HSA-free Eprex in Europe, given the concern over PRCA with this formulation and the lack of an alternative. In Canada, health care professionals have access to an alternative Eprex formulation (containing HSA and not polysorbate-80), so a “ban” on the product is not appropriate.
Sibbald also stated that Eprex has been banned in Australia. However, a “Dear Healthcare Professional” letter, issued by the sponsor in December 2002, recommends “that Eprex be given by the intravenous route where feasible, as this is thought to reduce the risk of antibody formation.”4 Therefore, to date, Eprex has not in fact been banned by the Australian Therapeutic Goods Administration.
It should also be noted that PRCA is not always irreversible; only 25% to 50% of patients become transfusion-dependent, and immunosupressive therapy can be effective in treating the condition.3
An alternative erythropoietin has not been on the Canadian market for long, and therefore the cumulative safety data are less extensive than for older products such as Eprex. In addition, many patients who currently take the newer product have also been exposed to Eprex. The limitation of assessing products that are relatively new to the market, on a background of exposure to another similar product, must be weighed against any safety considerations. Systematic efforts are being made by aca-demic researchers, Health Canada and the pharmaceutical industry to better define and address the problems of PRCA.
The importance of reporting adverse reactions to Health Canada or the manufacturer cannot be overstated. Health care practitioners are encouraged to familiarize themselves with the guidelines and mechanisms for adverse reaction reporting (see the Web site of the Canadian Adverse Reaction Monitoring Program, www.hc-sc.gc.ca/hpfb-dgpsa/tpd-dpt/index_adverse_e.html).
Carole Légaré Acting Director, Marketed Biologicals Biotechnology Products Division Christoper Turner Director General Marketed Health Products Directorate Anges V. Klein Manager Clinical Evaluation Division Biologics and Genetics Therapies Directorate Health Canada Ottawa, Ont.