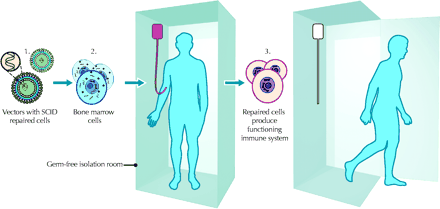
Until recently, a trial at Hôpital Necker Enfants Malades in Paris involving children with X-linked severe combined immunodeficiency disease (X-SCID, also known as “bubble-baby syndrome”) stood as the only unequivocal success story in gene transfer research. In the Paris trial, which began in 1999, a total of 11 boys with X-SCID (and without compatible donors) received transplants of their own bone marrow cells corrected by ex-vivo gene transfer using a retroviral vector to transduce the cells (see Fig). Initial reports indicated that the gene transfer had worked. However, the story abruptly changed on Oct. 3, 2002, when it was announced that 1 of the 9 children with a restored immune system had developed a leukemia-like condition characterized by uncontrollable proliferation of one particular type of Tcell. Three months later a second child developed a leukemia-like disease.1 Gene therapy appeared to be in crisis, and regulatory bodies around the world took emergency action.
Figure 1. Fundamentals of gene therapy. (1) Normal DNA is isolated and packaged into a vector (usually a disabled virus). (2) A target cell, usually from a tissue affected by the illness, is then infected with the vector. (3) The vector unloads its DNA, which then begins to produce the protein that was missing because of the gene defect, thus reversing the disease process. Adapted from www.fda.gov/fdac/features/2000/gene.html Photo: Chesley Sheppard
X-SCID is the most common genetic form of SCID, a rare and fatal immune disorder. Children with X-SCID have no working immune system (no white blood cells) and so are at risk of severe, recurrent infections. Typically, these children die within the first year of life unless they are treated successfully by allergenic bone marrow transplantation from a healthy donor with identical human leukocyte antigens. Nine of the 11 children in the Paris trial showed restored immune systems and sustained clinical benefit after gene transfer; they were able to go home and begin living relatively normal lives.
The setback to the Paris trial immediately raised the fear that the gene transfer had caused the leukemia. Indeed, in time the research team was able to confirm that the retroviral vectors carrying the missing gene had become integrated into chromosome 11, close to an oncogene (LMO-2) that is frequently associated with leukemia.1 (Since then, both children have responded well to chemotherapy and are currently in complete clinical remission.) At the time of the trial, it was generally believed that retroviral vectors carrying corrected or missing genes could insert themselves at random in the chromosomes of the cells they entered. It followed that there was a theoretical risk that vectors could become inserted near genes that regulate cellular growth and could thus contribute to cancerous changes in cells (a risk known as “insertional mutagenesis”). With the appearance of the 2 leukemia cases, this theoretical risk became very real.2
In response to the first case of leukemia, the Paris research team halted the trial. German, Italian and American regulatory authorities also put similar gene transfer trials on hold. The United Kingdom heightened their usual ongoing scrutiny of trials, adding case-by-case review of all potential new child research participants and a requirement for full disclosure of the increased risk of leukemia. After news of the second leukemia case, the US Food and Drug Administration convened a group of expert advisors, who voted 19 to 1 that gene transfer studies using retroviral vectors (27 such trials were ongoing in the United States at the time) be allowed to proceed if there were no acceptable alternative therapies for trial participants — which, in the case of X-SCID, would include children who had failed to find compatible bone marrow donors and would otherwise die. Fourteen trials in progress in this country were not halted by Health Canada, since they were using different gene transfer techniques.
The swift international reaction to the adverse event reports from Paris demonstrates a genuine concern for trial participants on the part of researchers and regulators worldwide. In the past decade, gene transfer researchers have been severely criticized for underestimating the time and money necessary to bring therapeutic gene products to market while also overestimating the short-term therapeutic potential of gene transfer,3 exposing clinical trial participants to serious risk in the face of adverse data, omitting important information from informed consent forms, and having financial conflicts of interest.4
The decision to halt gene transfer trials in the face of disheartening evidence about the risk of leukemia no doubt rested in part on a complex harm–benefit analysis. This analysis involved weighing the risk of X-SCID and imminent death against both the risk of leukemia (and possible death) and the potential benefit of gene transfer, for which there are no, or limited, therapeutic alternatives. One could argue that the boys enrolled in the Paris trial who developed leukemia were alive to do so only because they had participated in the trial. Some might argue that these children are actually worse off as a result of their participation in the current trial (there are, after all, better and worse ways of dying). Also, focusing on the interests of future children with X-SCID, one might argue that more research to develop safer gene transfer vectors should precede any further enrolment of children in gene transfer trials. The balance of harms and benefits in research involving humans is always a delicate issue, but is further complicated when the research participants are very sick, young children.
For now, it appears that the emerging consensus among regulators of gene transfer research is, “Proceed with caution.” In some jurisdictions, most notably the United Kingdom, this has meant continuing trials with increased scrutiny. In other jurisdictions it has meant a temporary hold on research to allow time to gather and evaluate new information, to re-evaluate the ethical acceptability of the research, to amend consent forms and processes, and perhaps even to allow for changes in the design of the vector. In both cases, the limited alternatives for these dying children motivated efforts to continue with gene transfer research.
The Paris leukemia cases remain the only known instances of insertional mutagenesis causing cancer in humans, and experts speculate that the leukemia may have been both disease- and protocol-specific.1 Regulators and scientists involved in gene transfer research are carefully monitoring research participants and are aware of the risk of cancer, at least with some kinds of gene transfer research. As to the final, long-term outcome of the X-SCID research, only time will tell.
Josephine Johnston Associate for Ethics, Law and Society The Hastings Center Garrison, NY Françoise Baylis Bioethics Department Dalhousie University Halifax, NS
Footnotes
-
We thank members of Dalhousie University's Novel Genetic Technologies Research Team, Josephine Nalbantoglu of McGill University and Karen Maschke of the Hastings Center for their feedback. Our thanks also to the Centre de Recherche en Ethique de L'Université de Montréal, where Françoise Baylis is Visiting Fellow for 2003-2004. This research is funded in part by a grant from the Canadian Institutes of Health Research.