Abstract
Background: Available evidence indicates that the use of antipsychotics, especially second-generation antipsychotics (SGAs), for children with mental health disorders has increased dramatically. Given the demonstrated metabolic and neurological adverse effects seen in adult patients on these medications, detailed evaluation of the risk for these adverse effects in children is appropriate.
Objective: The aim of the study was to assess the evidence for specific metabolic and neurological adverse effects associated with the use of SGAs in children.
Data Sources: MEDLINE (1996–May 2010) and EMBASE (1996–May 2010) databases were searched using highly sensitive search strategies for clinical trials in a paediatric population (children up to age 18 years).
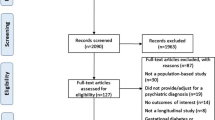
Study Selection: We included any double-blind, randomized controlled trial (RCT) of SGA medications conducted specifically in a paediatric population for the treatment of a mental health disorder. This included the medications risperidone, olanzapine, quetiapine, aripiprazole, clozapine, ziprasidone and paliperidone. The primary outcomes assessed for this review were metabolic and neurological adverse effects, as measured using physical examination manoeuvres, rating scales or laboratory tests. A total of 35 RCTs were included in the analysis, but not all studies had data that could be used in the meta-analysis.
Data Extraction: Abstracts retrieved from the searches were reviewed independently by two different reviewers for potential relevant articles. Full-text articles were then read in detail independently by two different reviewers to see if inclusion criteria were fulfilled. Data were extracted independently by two review authors from included studies and entered onto pre-designed summary forms. Clinical trials were evaluated for methodological quality using quality criteria developed by the US Preventive Services Task Force. Based on the fulfilment of quality criteria, studies were rated as good, fair or poor.
Data Synthesis: Meta-analysis was performed on the data for synthesis, and was carried out for commonly reported outcomes for each medication individually, in comparison with placebo or another drug. Odds ratios (ORs) with 95% confidence intervals for binary outcomes were used. For continuous outcomes, mean differences were used to analyze the data. Meta-analysis revealed that mean weight gain compared with placebo was highest for olanzapine at 3.47 kg (95% CI 2.94, 3.99) followed by risperidone at 1.72kg (95% CI 1.17, 2.26), quetiapine at 1.41kg (95% CI 1.10, 1.81) and aripiprazole at 0.85 kg (95% CI 0.58, 1.13). Olanzapine and clozapine treatment were associated with the highest rate of metabolic laboratory abnormalities in cholesterol and triglycerides. Prolactin elevation occurred with risperidone and olanzapine therapy. Higher odds of extrapyramidal symptoms compared with placebo were seen in children treated with risperidone (OR 3.55; 95% CI 2.04, 5.48) and aripiprazole (OR 3.70; 95% CI 2.37, 5.77). Elevated rates of extrapyramidal symptoms were also experienced with olanzapine use.
Conclusions: There is good evidence to support the existence of both metabolic and neurological adverse effects in children treated with these medications. Proper attention and vigilance to potential metabolic and neurological adverse effects is necessary, and should be considered part of the standard of care.

















Similar content being viewed by others
References
Leucht S, Pitschel-Walz G, Abraham D, et al. Efficacy and extrapyramidal side effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo: a meta-analysis of randomized controlled trials. Schizophr Res 1999; 35: 51–68
Janno S, Holi M, Tuisku K, et al. Prevalence of neuroleptic induced movement disorders in chronic schizophrenia in-patients. Am J Psychiatry 2004; 161: 160–3
DelBello M, Schwiers M, Rosenberg L, et al. A double-blind, randomized, placebo-controlled study of quetiapine as adjunctive treatment for adolescent mania. J Am Acad Child Adolesc Psychiatry 2002; 41(10): 1216–23
Findling R, Robb A, Nyilas M, et al. A multiple-center, randomized, double-blind, placebo-controlled study of oral aripiprazole for treatment of adolescents with schizophrenia. Am J Psychiatry 2008; 11(165): 1369–72
Kryzhanovskaya L, Schulz S, McDougle C, et al. Olanzapine versus placebo in adolescents with schizophrenia: a 6-week randomized, double-blind, placebo-controlled trial. J Am Acad Child Adolesc Psychiatry 2009; 48(1): 60–70
Kumra S, Frazier J, Jacobsen L, et al. Childhood-onset schizophrenia: a double-blind clozapine-haloperidol comparison. Arch Gen Psychiatry 1996; 53(12): 1090–7
Marcus R, Owen R, Kamen L, et al. A placebo-controlled, fixed-dose study of aripiprazole in children and adolescents with irritability associated with autistic disorder. J Am Acad Child Adolesc Psychiatry 2009; 48(11): 1110–9
Sallee F, Kurlan R, Goetz C, et al. Ziprasidone treatment of children and adolescents with tourette’s syndrome: a pilot study. J Am Acad Child Adolesc Psychiatry 2000; 39(3): 292–9
Haas M, Unis A, Armenteros J, et al. A 6-week, randomized, double-blind, placebo-controlled study of the efficacy and safety of risperidone in adolescents with schizophrenia. J Child Adolesc Psychopharmacol 2009; 19(6): 611–21
Patel N, Sanchez R, Johnsrud M, et al. Trends in antipsychotic use in Texas Medicaid population of children and adolescents: 1996 to 2000. J Child Adolesc Psychopharmacol 2002; 12(3): 221–9
Olfson M, Blanco C, Liu L, et al. National trends in the outpatient treatment of children and adolescents with antipsychotic drugs. Arch Gen Psychiatry 2006; 63: 679–85
Newcomer J. Second generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs 2005; 19 Suppl. 1: 1–93
Correll C, Manu P, Olshanskiy V, et al. Cardiometabolic risk of second generation antipsychotic medications during first time use in children and adolescents. JAMA 2009; 302(16): 1765–73
Miller D, Caroff S, Davis S, et al. Extrapyramidal side effects of antipsychotics in a randomized trial. Br J Psychiatry 2008; 193(4): 279–88
Woods SW, Martin A, Spector SG, et al. Effects of development on olanzapine-associated adverse events. J Am Acad Child and Adolesc Psychiatry 2002 Dec; 41(12): 1439–46
Harris RP, Helfand MH, Woolf SH, et al. Current methods of the U.S. Preventive Services Task Force. Am J Prev Med 2001; 20 (3 Suppl.): 22–35
Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327(7414): 557–60
Research Units on Pediatric Psychopharmacology (RUPP) Autism Network. Risperidone in children with autism and serious behavioural problems. N Engl J Med 2002; 347(5): 314–21
Shea S, Turgay A, Carroll A, et al. Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics 2004; 114(5): e634–41
Snyder R, Turgay A, Aman M, et al. Effects of risperidone on conduct and disruptive behaviour disorders in children with subaverage IQs. The Risperidone Conduct Study Group. J Am Acad Child Adolesc Psychiatry 2002; 41(9): 1026–36
Aman M, De Smedt G, Derivan A, et al. Double-blind, placebo-controlled study of risperidone for the treatment of disruptive behaviors in children with subaverage intelligence. Risperidone Disruptive Behavior Study Group. Am J Psychiatry 2002; 159(8): 1337–46
Van Bellinghen M, De Troch C. Risperidone in the treatment of behavioural disturbances in children and adolescents with borderline intellectual functioning: a double-blind, placebo-controlled pilot trial. J Child Adolesc Psychopharmacol 2001; 11(1): 5–13
Findling R, McNamara N, Branicky L, et al. A double-blind pilot study of risperidone in the treatment of conduct disorder. J Am Acad Child Adolesc Psychiatry 2000; 39(4): 509–16
Buitelaar J, van der Gaag R, Cohen-Kettenis P, et al. A randomized controlled trial of risperidone in the treatment of aggression in hospitalized adolescents with subaverage cognitive abilities. J Clin Psychiatry 2001; 62(4): 239–48
Armenteros J, Lewis J, Davalos M. Risperidone augmentation for treatment-resistant aggression in attention-deficit/ hyperactivity disorder: a placebo-controlled pilot study. J Am Acad Child Adolesc Psychiatry 2007; 26(5): 558–65
Haas M, DelBello N, Pandina G, et al. Risperidone for the treatment of acute mania in children and adolescents with bipolar disorder: a randomized, double-blind, placebo-controlled study. Bipolar Disord 2009; 11(7): 687–700
Reyes M, Buitelaar J, Toren P, et al. A randomized, double-blind, placebo-controlled study of risperidone maintenance treatment in children and adolescents with disruptive behaviour disorders. Am J Psychiatry 2006; 163(3): 402–10
Sikich L, Hamer R, Bashford R, et al. A pilot study of risperidone, olanzapine, and haloperidol in psychotic youth: a double-blind, randomized, 8-week trial. Neuropsychopharmacology 2004; 29(1): 133–45
Sikich L, Frazier J, McClellan J, et al. Double-blind comparison of first- and second-generation antipsychotics in early-onset schizophrenia and schizoaffective disorder: findings from the Treatment of Early-Onset Schizophrenia Spectrum disorders (TEOSS) study. Am J Psychiatry 2008; 165(11): 1420–31
Haas M, Eerdekens M, Kushner S, et al. Efficacy, safety and tolerability of two risperidone dosing regiments in adolescent schizophrenia: double-blind study. Br J Psychiatry 2009; 194(2): 158–64
Gilbert D, Batterson J, Sethuraman G, et al. Tic reduction with risperidone versus pimozide in a randomized, double-blind, crossover trial. J Am Acad Child Adolesc Psychiatry 2004; 43(2): 206–14
Gaffney G, Perry P, Lund B, et al. Risperidone versus clonidine in the treatment of children and adolescents with tourette’s syndrome. J Am Acad Child Adolesc Psychiatry 2002; 41(3): 330–6
Nagaraj R, Singhi P, Malhi P. Risperidone in children with autism: randomized, placebo-controlled, double-blind study. J Child Neurol 2006; 21(6): 450–5
Luby J, Mrakotsky C, Stalets M, et al. Risperidone in preschool children with autistic spectrum disorders: an investigation of safety and efficacy. J Child Adolesc Psychopharmacol 2006; 16(5): 575–87
Miral S, Gencer O, Inal-Emiroglu F, et al. Risperidone versus haloperidol in children and adolescents with AD: a randomized, controlled, double-blind trial. Eur Child Adolesc Psychiatry 2008; 17(1): 1–8
Tohen M, Kryzhanovskaya L, Carlson G, et al. Olanzapine versus placebo in the treatment of adolescents with bipolar mania. Am J Psychiatry 2007; 164(10): 1547–56
Hollander E, Wasserman S, Swanson E, et al. A double-blind placebo-controlled pilot study of olanzapine in childhood/adolescent pervasive development disorder. J Child Adolesc Psychopharmacol 2006; 16(5): 541–8
DelBello M, Chang K, Welge J, et al. A double-blind, placebo-controlled pilot study of quetiapine for depressed adolescents with bipolar disorder. Bipolar Disord 2009; 11(5): 483–93
Connor D, McLaughlin T, Jeffers-Terry M. Randomized controlled pilot study of quetiapine in the treatment of adolescent conduct disorder. J Child Adolesc Psychopharmacol 2008; 18(2): 140–56
DelBello M, Kowatch R, Adler C, et al. A double blind randomized pilot study comparing quetiapine and divalproex for adolescent mania. J Am Acad Child Adolesc Psychiatry 2006; 45(3): 305–13
Owen R, Sikich L, Marcus R, et al. Aripiprazole in the treatment of irritability in children and adolescents with autistic disorder. Pediatrics 2009; 124(6): 1533–40
Findling R, Nyilas M, Forbes R, et al. Acute treatment of pediatric bipolar I disorder, manic or mixed episode, with aripiprazole: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry 2009; 70(10): 1441–51
Tramontina S, Zeni C, Ketzer C, et al. Aripiprazole in children and adolescents with bipolar disorder comorbid with attention-deficit/hyperactivity disorder: a pilot randomized clinical trial. J Clin Psychiatry 2009; 70(5): 756–64
Shaw P, Sporn A, Gogtay N, et al. Childhood-onset schizophrenia: a double-blind, randomized clozapine-olanzapine comparison. Arch Gen Psychiatry 2006; 63(7): 721–30
Kumra S, Kranzler H, Gerbino-Rosen G, et al. Clozapine and “high-dose” olanzapine in refractory early-onset schizophrenia: a 12-week randomized and double-blind comparison. Biol Psychiatry 2008; 63(5): 524–9
Mattson N, Ronnemaa T, Juonala M, et al. Childhood predictors of the metabolic syndrome in adulthood: the cardiovascular risk in young finns study. Ann Med 2008; 40: 542–52
Al Mamun A, Cramb S, O’Callaghan M, et al. Childhood overweight status predicts diabetes at age 21 years: a follow-up study. Obesity (Silver Spring) 2009; 17(6): 1255–61
Juonala M, Jarvisalo M, Maki-Torkko N, et al. Risk factors identified in childhood and decreased carotid artery elasticity in adulthood. Circulation 2005; 112: 1486–93
Pulkki-Raback L, Elovainio M, Kivimaki M, et al. Depressive symptoms and the metabolic syndrome in childhood and adulthood: a prospective cohort study. Health Psychol 2009; 28(1): 108–16
Morato E, Druss B, Hartung D, et al. Metabolic testing rates in 3 state Medicaid programs after FDA warnings and ADA/APA recommendations for second generation antipsychotic drugs. Arch Gen Psychiatry 2010; 67(1): 17–24
Acknowledgements
Funding for this work was provided by the Canadian Institute of Health Research. The funding organization was not involved in any aspect of research design or manuscript preparation.
Tamara Pringsheim, Darren Lam, Heidi Ching and Scott Patten do not have any potential conflicts of interest in relation to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Pringsheim, T., Lam, D., Ching, H. et al. Metabolic and Neurological Complications of Second-Generation Antipsychotic Use in Children. Drug-Safety 34, 651–668 (2011). https://doi.org/10.2165/11592020-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11592020-000000000-00000