-
PDF
- Split View
-
Views
-
Cite
Cite
Louis Valiquette, Benoit Cossette, Marie-Pierre Garant, Hassan Diab, Jacques Pépin, Impact of a Reduction in the Use of High-Risk Antibiotics on the Course of an Epidemic of Clostridium difficile-Associated Disease Caused by the Hypervirulent NAP1/027 Strain, Clinical Infectious Diseases, Volume 45, Issue Supplement_2, September 2007, Pages S112–S121, https://doi.org/10.1086/519258
Close - Share Icon Share
Abstract
A series of measures were implemented, in a secondary/tertiary-care hospital in Quebec, to control an epidemic of nosocomial Clostridium difficile-associated disease (n-CDAD) caused by a virulent strain; these measures included the development of a nonrestrictive antimicrobial stewardship program. Interrupted time-series analysis was used to evaluate the impact of these measures on n-CDAD incidence. From 2003–2004 to 2005–2006, total and targeted antibiotic consumption, respectively, decreased by 23% and 54%, and the incidence of n-CDAD decreased by 60%. No change in n-CDAD incidence was noted after strengthening of infection control procedures (P = .63), but implementation of the antimicrobial stewardship program was followed by a marked reduction in incidence (P = .007). This suggests that nonrestrictive measures to optimize antibiotic usage can yield exceptional results when physicians are motivated and that such measures should be a mandatory component of n-CDAD control. The inefficacy of infection control measures targeting transmission through hospital personnel might be a result of their implementation late in the epidemic, when the environment was heavily contaminated with spores.
Clostridium difficile-associated disease (CDAD) is the leading cause of nosocomial diarrhea in industrialized countries. Since the end of 2002, >30 hospitals in the province of Quebec, Canada, have been struggling with an epidemic of CDAD characterized by a high case-fatality rate [1, 2] and an increased risk of recurrence after metronidazole treatment [3]. In Sherbrooke, Quebec, the incidence among individuals ⩾65 years of age increased from 102 cases/100,000 population in 1991–1992 to 866 cases/100,000 population in 2003 [1]. This epidemic has been associated with an emerging strain, NAP1/027, characterized by toxin A and B hyperproduction [4]. Outbreaks due to this same clone have been reported in the United States, the United Kingdom, France, Belgium, and The Netherlands [5].
Receiving antibiotics in the preceding weeks is the most important risk factor for CDAD. Historically, CDAD was associated with a variety of antibiotics, especially third-generation cephalosporins, ampicillin, and clindamycin [6]. Recently, an association between the NAP1/027 clone and fluoroquinolone usage has been consistently reported [7–9]. Before this epidemic, several authors documented how actively restricting the use of high-risk antibiotics led to a reduction in the incidence of CDAD caused by strains other than NAP1/027 [10–12]. As a result, minimizing the inappropriate use of antibiotics is considered to be an important element of CDAD control [13]. A series of measures were implemented at our institution to control the CDAD epidemic, including the development of a nonrestrictive antibiotic control program. To evaluate their impact, we undertook a retrospective analysis of antibiotic consumption before, during, and after the peak epidemic period.
Methods
Population and surveillance periods. Centre Hospitalier Universitaire de Sherbrooke (CHUS), in the province of Quebec, Canada, is a 683-bed, secondary/tertiary-care hospital located at 2 distinct sites (Hôpital Fleurimont and Hôpital Hôtel-Dieu). We conducted retrospective surveillance of antibiotic usage and CDAD incidence among all hospitalized patients from January 2003 to March 2006. Patients <18 years of age and those hospitalized in psychiatric wards were excluded; the former were not targeted by our guidelines, and the latter rarely receive antibiotics. Data were compiled for 4-week periods based on the government of Quebec's fiscal calendar, which begins on 1 April.
CDAD incidence. Patients with a positive toxin assay result were identified through the hospital's computerized medical records (Misys Healthcare Systems). Additionally, for inpatients, cases of pseudomembranous colitis, antibiotic-associated colitis, or C. difficile colitis were identified through the hospital discharge database. Patients were considered to have developed CDAD if they met at least 1 of the following criteria: a positive C. difficile cytotoxin assay result, endoscopic evidence of pseudomembranous colitis, or histopathologic evidence of pseudomembranous colitis in a biopsy specimen obtained during colonoscopy, colectomy, or autopsy [1]. Two episodes of CDAD in the same patient were considered to be distinct events if they occurred ⩾2 months apart; an episode that occurred within 2 months after a prior episode was considered to be a recurrence and was not included in measures of incidence. CDAD was defined as nosocomial (n-CDAD) if the patient had been hospitalized for ⩾24 h within the previous 2 months. n-CDAD incidence is expressed as the number of cases per 1000 patient-days of hospitalization. In retrospect, there had been a slight increase in n-CDAD incidence at the end of 2002, but the epidemic was recognized in the middle of 2003 [14], with the peak incidence occurring from July 2003 to November 2004.
Interventions. The first measure taken, in September and October 2003, to reduce n-CDAD incidence was to strengthen infection control procedures through several interventions: (1) staff education, (2) strict isolation for patients with diarrhea (before results of C. difficile assay were available and, for patients with positive results, until discharge), (3) the use of dedicated equipment with disposable rectal thermometers, and (4) environmental cleaning with hypochlorite sodium. In December 2003, room disinfection procedures were further strengthened through (1) additional personnel education, (2) sodium hypochlorite being replaced by 7% accelerated hydrogen peroxide (Virox Technologies) for terminal disinfection of rooms of patients with CDAD, and (3) comprehensive ward disinfection with sodium hypochlorite when ⩾3 nosocomial cases were noted on any given ward. Personnel education sessions on isolation, disinfection, and cleaning procedures were conducted repetitively throughout this period.
Unfortunately, these measures did not have a measurable impact on n-CDAD incidence during the winter of 2003–2004, prompting a second major intervention to optimize antimicrobial prescribing. To this end, local guidelines were developed by infectious diseases physicians and pharmacists and publicized initially by distributing (in February 2004) a letter to all physicians and pharmacists, followed by oral presentations to selected services, and by releasing (in October 2004) a pocket-sized antibiotic guide focusing on empirical treatment of common infections necessitating hospital admissions. These guidelines aimed to decrease the use of antibiotics most commonly associated with CDAD in our institution in 2003 [1]: second- and third-generation cephalosporins, ciprofloxacin, clindamycin, and macrolides ("targeted antibiotics"). No formal restriction was applied, but recommendations were reinforced through telephone feedback by pharmacists to physicians to suggest alternatives when applicable. Examples of recommendations included (1) favoring gentamicin instead of ciprofloxacin for acute pyelonephritis in patients with a normal renal function, (2) using cotrimoxazole rather than ciprofloxacin for lower urinary tract infection caused by susceptible pathogens, (3) replacing the cephalosporin/azithromycin combination with a respiratory fluoroquinolone (initially levofloxacin, later moxifloxacin) for community-acquired pneumonia, (4) favoring gentamicin/metronidazole or piperacillin/tazobactam over ciprofloxacin/metronidazole for intra-abdominal infections, and (5) avoiding clindamycin, except in patients with severe allergy to β-lactams, streptococcal toxic shock syndrome, or necrotizing infections. Following Infectious Diseases Society of America guidelines, we recommended that the duration of treatment be shortened as follows: (1) to 8 days for hospital-acquired pneumonia (when caused by pathogens other than Pseudomonas aeruginosa and Acinetobacter species), (2) to 3 days after the patient became afebrile for pneumococcal pneumonia, and (3) to until the resolution of clinical signs and normalization of leucocytosis for intra-abdominal infections [15].
Antibiotic utilization. Antibiotic consumption data were extracted from a clinical data warehouse (Centre Informatisé de Recherche Évaluative en Services et Soins de Santé [CIRESSS]) combining extensive data from the computerized patients' records with data on diagnoses (International Classification of Diseases, Ninth Revision, Clinical Modification). Only agents with antibacterial activity were included in our review and were termed “antibiotics” in the present article. Prescriptions written by physicians are entered into computerized patient records by pharmacy technicians and validated by pharmacists. This system has been used at CHUS Hôpital Fleurimont for several years but became fully functional at CHUS Hôpital Hôtel-Dieu only at the end of 2002; therefore, data on antibiotic prescriptions were collected starting in January 2003. Our level of computerization allowed us to calculate data on antibiotic consumption directly from patients' prescription profiles and to calculate the prescribed daily dose (PDD) rather than extrapolating from the amount dispensed by pharmacies divided by a “defined daily dose” (DDD) proposed by the World Health Organization [16]. Antibiotic consumption was calculated in patient-days (the number of days during which a patient is receiving a specific antibiotic). When, on a given day, a patient received 2 (or more) different antibiotics, it was computed as 2 (or more) patient-days. The summary measure for overall and targeted antibiotics was further expressed as a fraction, the denominator being patient-days of hospitalization during the corresponding period. We propose to designate this fraction as the antibiotic utilization ratio (AUR).
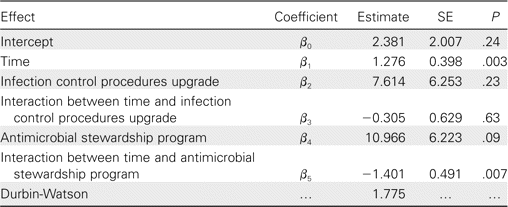
Statistical analysis. Segmented regression analysis for interrupted time series was used to determine the significance of the differences in levels and slopes over time due to (1) infection control strengthening and (2) antibiotic optimization. This type of analysis provides a methodologically acceptable alternative for the assessment of long-term effects on an outcome attributable to a specific intervention [17, 18]. The level and trend of the preintervention segment serve as controls for the postintervention segment. The interrupted time-series analysis in our model can be specified as E(Y) = β0 + β1t + β2x1 + β3tx1 + β4x2 + β5tx2, where Yt is the dependent variable (n-CDAD), t indicates the order (fiscal periods) in which the observations were taken, and x1 and x2 are binary dummy variables indicating whether the observation was obtained before or after the intervention. Two distinct variables were necessary to evaluate 2 different interventions. β3 and β5, respectively, represent the change in n-CDAD incidence over time (slope) before and after infection control practices upgrade and antibiotic optimization. The Durbin-Watson statistic was used to test for autocorrelation in the residuals (range, 0–4; midpoint, 2); a value of 2.0 indicated that a serial correlation was not detected [19]. Significance was determined at the .05 level, and SAS version 9.0 (SAS) was used for statistical programming.
Results
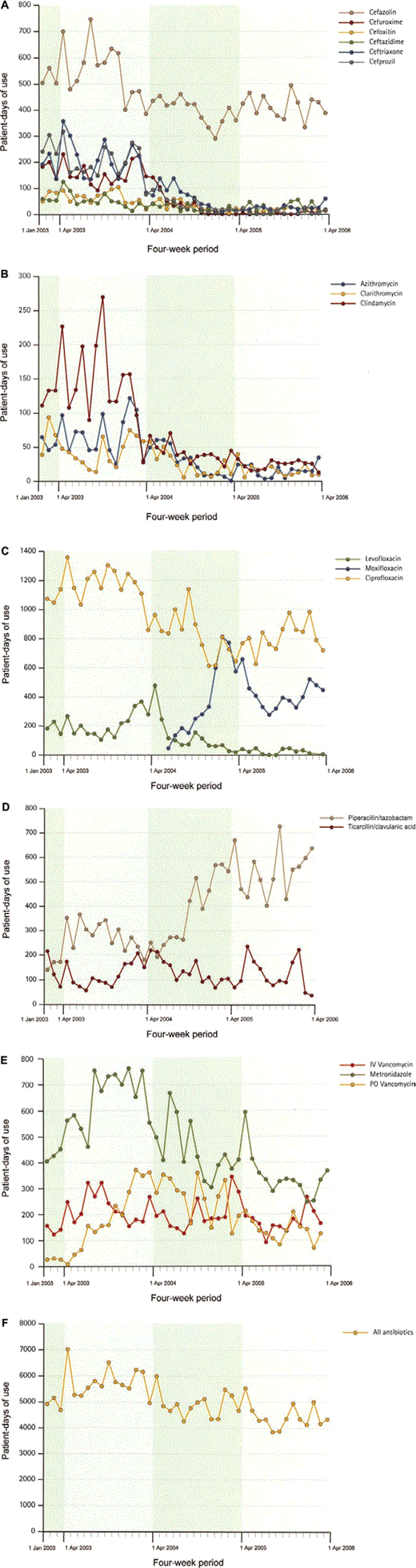
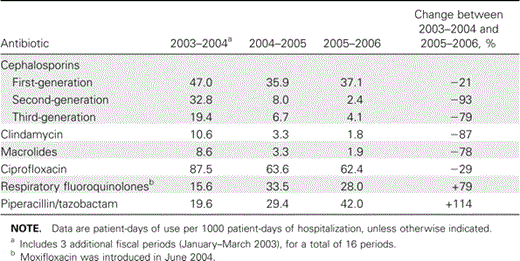
Impact of guidelines on antibiotic usage. Figure 1A–1D and table 1 show the impact of guidelines on the use of targeted antibiotics. Important decreases were noted for all cephalosporins, but the impact on second- and third-generation cephalosporins was higher. The reduction was so important that, for certain periods of 2005–2006, there was no consumption of cefuroxime. Although first-generation cephalosporins were not targeted, a reduction of 21% was noted in their use between 2003–2004 and 2005–2006. There were dramatic reductions in the use of clindamycin and macrolides and a 29% reduction in the use of ciprofloxacin. Predictably, these changes were associated with increases in the consumption of antibiotics recommended as alternatives: moxifloxacin (as a substitute for second- or third-generation cephalosporins with or without macrolides for treatment of community-acquired pneumonia) (figure 1C) and piperacillin/tazobactam (to replace ciprofloxacin/metronidazole for abdominal/pelvic infections) (figure 1D). Figure 1E shows the change over time in the use of metronidazole (some of which was to treat CDAD), intravenous vancomycin (unrelated to CDAD), and oral vancomycin (used only against CDAD).
Antibiotic consumption in patient-days, 2003–2006. A, Cephalosporins. B, Clindamycin and macrolides. C, Fluoroquinolones. D, β-Lactam/β-lactamase inhibitors. E, Metronidazole and vancomycin. F, Total antibiotic consumption.
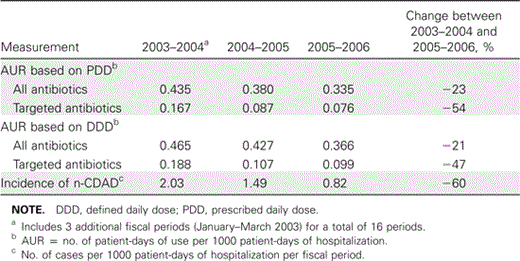
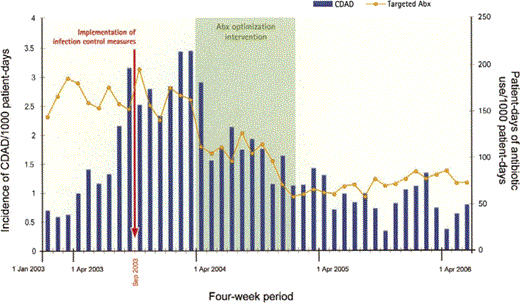
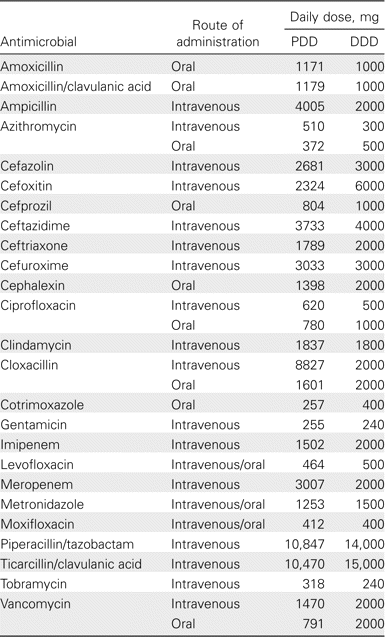
Antibiotic use and n-CDAD incidence. A striking consequence of local guideline implementation was a durable decrease in the overall consumption of antibiotics (figure 1F). During the period of surveillance, total antibiotic and targeted antibiotic consumption, respectively, decreased by 23% and 54%, and n-CDAD incidence decreased by 60% (table 2). A decrease in the use of targeted antibiotics supervened shortly after the antimicrobial stewardship intervention and was congruent with the reduction in n-CDAD incidence, the 2 curves being strikingly parallel (figure 2). This contrasts with the lack of a measurable impact on n-CDAD incidence after upgrading infection control procedures. Table 2 shows the changes in the AUR, measured using the PDD and the DDD. To explain the discrepancies between the 2 methods, especially for targeted antibiotics, the actual PDD and DDD for selected drugs are shown in table 3.
Overall and targeted antibiotic utilization ratios (AURs) and incidence of nosocomial Clostridium difficile-associated disease (n-CDAD), 2003–2006.
Targeted antibiotic (Abx) consumption and nosocomial Clostridium difficile-associated disease (CDAD) incidence per 1000 patient-days of hospitalization.
Prescribed daily dose (PDD) and World Health Organization-defined daily dose (DDD) for frequently used antibiotics.
Interrupted time-series analysis. No significant decrease in n-CDAD incidence was noted after the implementation of infection control procedures (P = .63), but implementation of the antimicrobial stewardship program was followed by a negative change (P = .007) (table 4). Most changes in infection control procedures occurred during the first part of the outbreak, and few notable modifications were introduced after the antimicrobial stewardship program.
Interrupted time-series analysis evaluating relationships between nosocomial Clostridium difficile-associated disease and time.
Discussion
Control of n-CDAD is a complex issue. Most authors suggest a multifaceted approach aiming to interrupt transmission between individuals and to reduce the likelihood of clinical illness in patients with C. difficile colonization [13, 20]. How to translate these general principles into practice remains debated, because most potential measures are not supported by solid evidence. Only 3 interventions are evidence based: policies for prudent use of antibiotics, gloves for contact with infected patients, and disposable, single-use thermometers [21]. Consequently, interventions vary substantially between countries and institutions. In our center, initial efforts focused on horizontal transmission by hospital personnel. Although these measures were congruent with standard infection control practices, actively reinforced, and implemented just before the peak incidence of n-CDAD, they had no significant impact during the following months, perhaps to some extent because compliance with such measures is difficult to sustain. Furthermore, we speculate that, by the time infection control measures were strengthened, there had already been massive dissemination of C. difficile spores throughout the hospital environment; at this stage of the epidemic, the hands of health care providers may have been less important in transmission than was acquisition of the pathogen from contaminated environmental surfaces. There is an obvious need for further research addressing the relative importance of these 2 modes of C. difficile transmission during epidemics. As in most hospitals in North America, our routine surveillance of nosocomial infections did not include n-CDAD before 2003. Arguably, if infection control measures had been strengthened earlier on, when the environment was less contaminated, such efforts might have been more fruitful. Given the design of this study, it is impossible to prove that a decrease in antibiotic consumption alone might have led to the same results. Infection control procedures were started before and continued throughout the implementation of our antimicrobial stewardship program. Consequently, one should not interpret our results as a dismissal of infection control procedures. However, our results convincingly demonstrate that antimicrobial stewardship programs should be an obligatory element of CDAD control.
Implementation of a nonrestrictive antimicrobial stewardship program was associated with stunning decreases in the use of high-risk antibiotics and a significant decrease in overall antibiotic use, followed by a rapid and durable decrease in n-CDAD incidence. Evaluating hospital-wide interventions is difficult. Because of organizational issues, high costs, and lack of power, cluster-randomized trials comparing centers instead of individuals are rarely performed. Consequently, researchers rely on quasi-experimental designs, which often examine the effect of introducing or removing a single antibiotic from a hospital formulary [22]. Such findings need to be interpreted with caution: given the seasonal variation in n-CDAD incidence [23], a new antibiotic might be seen as worsening the situation if it is introduced in September and as being protective if it is introduced in April. Comparison of pre- and postintervention slopes, using segmental interrupted time series, allows the estimation of secular changes resulting from maturation or regression to the mean before the intervention is implemented and adjustment for autocorrelation. The use of repeated measurements before and after the intervention should also allow testing and instrumentation effects to be controlled. Time-series analyses do not establish causality between a given intervention and a subsequent, statistically significant change in the incidence of a disease. In this case, however, causality seems highly plausible, considering (1) the well-established causal association between antibiotic exposure and CDAD, (2) the dramatic decrease in the use of antibiotics independently linked to CDAD in a local study [8], and (3) the parallel nature of the 2 curves shown in figure 2. After March 2005, the curves diverged somewhat, the decrease in CDAD incidence being more marked than the decrease in the use of targeted antibiotics. This might reflect an indirect effect of antibiotic optimization during the preceding year, with fewer cases leading to less exposure to C. difficile spores for subsequent cohorts of patients. In other words, the vicious circle was transformed into a virtuous circle.
The February 2004 recommendations on the selection of antimicrobials were based on data then available from a retrospective study that used univariate analyses to compare antibiotic exposure within 2 months before n-CDAD diagnosis and hospital consumption of the same antibiotics [1]. Better data became available later, from a cohort study of CHUS inpatients, which examined the relative risks of n-CDAD associated with various antibiotics after adjustment for important confounding factors, such as age, duration of hospitalization (a proxy for exposure), and concomitant administration of other antibiotics [8]. This analysis identified fluoroquinolones, especially ciprofloxacin, as the driving force of the epidemic. In retrospect, the recommendation to replace ceftriaxone/azithromycin with a respiratory fluoroquinolone for inpatients with community-acquired pneumonia was debatable. However, its overall effect was probably neutral, because the hazard ratio of the ceftriaxone/azithromycin combination (the product of the adjusted hazard ratio for each drug) was identical (adjusted hazard ratio, 2.57) to that of levofloxacin alone (adjusted hazard ratio, 2.52) [8].
Historically, antimicrobial stewardship interventions that were effective in controlling n-CDAD were restrictive and frequently focused on a single high-risk antibiotic [10, 24–27]. Our study suggests that passive, nonrestrictive, and simple measures to optimize antibiotic usage can have exceptional results when physicians are highly motivated, because potential adverse effects of antimicrobials supervene within a short time frame. This contrasts with other interventions for antibiotic stewardship, in which physicians are asked to sacrifice short-term, perceived or genuine personal gains (broad-spectrum coverage or more prolonged antimicrobial therapy thought to be more secure for a given patient and less likely to result in liability) for the sake of long-term benefits for the community in what is generally seen as a lost cause (avoidance of antimicrobial resistance).
Our data do not suggest that the staggering epidemic of CDAD in our hospital was precipitated by an aberrant overall level of antimicrobial use. Many researchers have measured the overall use of antibiotics among inpatients, dividing amounts of drugs dispensed out of hospital pharmacies by the DDD and relating this to the number of patient-days of hospitalization, and they have obtained remarkably similar results: the AUR was found to be 0.400 in Switzerland [28], 0.402 in France [29], 0.425–0.547 in The Netherlands [30, 31], 0.448 in Denmark [32], 0.477 in Sweden [33], 0.490 in German regional hospitals, and 0.601–0.793 in German university hospitals [34, 35]. This compares closely to our AUR estimate calculated using the same DDD: 0.465 in 2003–2004. If the building up of the epidemic is unexplained by quantitative differences, could qualitative differences in the selection of antimicrobials have played a role? As mentioned above, fluoroquinolones, especially ciprofloxacin, were associated with the highest relative risk for CDAD [8]. At CHUS in 2003–2004, fluoroquinolones contributed to 28% of the total AUR measured using the DDD (and 25% of that measured using the PDD), compared with 3% in France [29], 6% in Denmark [32], 10% in The Netherlands [31], and 15%–22% in Germany [35]. Thus, overuse of fluoroquinolones may have contributed to the magnitude of the n-CDAD epidemic.
The DDD method is meant to facilitate comparisons between hospitals and countries. As noted by others [35–37], for several antimicrobials, there were major discrepancies between the DDD and the actual PDD. For many drugs, the DDD is the most common dose used to treat patients with normal renal function (e.g., vancomycin, 2000 mg). In industrialized countries, the population of inpatients is aging considerably, resulting in an increase in the proportion of patients having renal dysfunction requiring adjustment of antimicrobials. For other drugs, the rationale behind the DDD is unclear. For instance, the DDDs for intravenous oxacillin and cloxacillin are both 2000 mg, but, in practice, patients with a severe staphylococcal infection rarely receive <6000 mg/day. The DDD for intravenous ciprofloxacin is 500 mg, whereas the drug is marketed as 400-mg vials, normally given twice a day. Furthermore, both prescribing habits and the case mix in a given hospital will have an impact on the PDD; for instance, in hospitals providing cardiac surgery and tertiary cardiology services, a higher proportion of patients with staphylococcal bacteremia have endocarditis as the source, leading to a higher PDD for oxacillin. Finally, antibiotics prescribed for perioperative prophylaxis (cefazolin and cefoxitin) end up with a PDD lower than the DDD.
In our case, estimating the overall use of antibiotics via the DDD produced results similar to those measured via the PDD, with the overestimates balancing the underestimates. However, estimating patients' exposure to specific antibiotics on the basis of the amount dispensed by pharmacies divided by the DDD would have grossly biased the results. Because more and more hospitals now benefit from computerized drug prescriptions, the DDD system may soon become obsolete and should be replaced with a direct measure of exposure to antibiotics via the PDD. This would allow more precise monitoring of hospital-wide antibiotic use and better assessment of the potential impact of various interventions.
Acknowledgments
We are indebted to Odette Favreau, for providing information on the infection control upgrading at Centre Hospitalier Universitaire de Sherbrooke during the study period.
Supplement sponsorship. This article was published as part of a supplement entitled “Annual Conference on Antimicrobial Resistance,” sponsored by the National Foundation for Infectious Diseases.
Potential conflicts of interest. L.V. has been on the speakers' bureau for Wyeth; has served on advisory boards for Wyeth and Cubist; and has received grants from Wyeth, Genzyme, and Arpida. J.P. has been on the speakers' bureau for Wyeth Canada; has served on advisory boards for Bayer, Wyeth, Viropharma, and Acambis; and has received grants from Genzyme. All other authors: no conflicts.





Comments